Glaesserella parasuis serotype 4 HPS4-YC disrupts the integrity of the swine tracheal epithelial barrier and facilitates bacterial translocation
- PMID: 34674760
- PMCID: PMC8529811
- DOI: 10.1186/s13567-021-01005-w
Glaesserella parasuis serotype 4 HPS4-YC disrupts the integrity of the swine tracheal epithelial barrier and facilitates bacterial translocation
Abstract
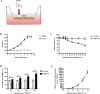
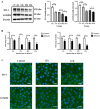
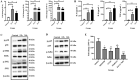
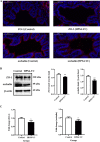
Glaesserella parasuis (G. parasuis) is a commensal bacterium in the upper respiratory tract of pigs that can also cause the swine Glässer disease, which induces an intensive inflammatory response and results in significant economic losses to the swine industry worldwide. G. parasuis can cause disease through infection of the respiratory tract, resulting in systemic infection, but the mechanism is largely unknown. Recently we showed that Glaesserella parasuis serotype 4 (GPS4) increased swine tracheal epithelial barrier permeability, resulting in easier bacterial translocation. Tight junction proteins (TJ) play a crucial role in maintaining the integrity and impermeability of the epithelial barrier. GPS4 decreased the expression of the TJ ZO-1 and occludin in swine tracheal epithelial cells (STEC). Furthermore, the proinflammatory cytokines IL-6, IL-8 and TNF-α were significantly upregulated in GPS4-infected STEC, and both the MAPK and NF-κB signaling pathways were activated and contributed to the expression of TNF-α. We demonstrate that the production of proinflammatory cytokines, especially TNF-α, during GPS4 infection was involved in barrier dysfunction. Additionally, animal challenge experiments confirmed that GPS4 infection downregulated TJ in the lungs of piglets and induced a severe inflammatory response. In general, G. parasuis infection downregulated the expression of TJ and induced massive secretion of proinflammatory cytokines, resulting in epithelial barrier disruption and favoring bacterial infection. This study allowed us to better understand the mechanism by which G. parasuis crosses the respiratory tract of pigs.
Keywords: Glaesserella parasuis; proinflammatory cytokines; swine tracheal epithelial cells; tight junction proteins.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Grants and funding
- 2017YFD0500203/the national key research and development program of china
- CX(19)2020/the jiangsu agricultural science and technology innovation fund
- rc392012/the research fund for introducing and stabilizing talents of anhui agricultural university
- PAPD/the priority academic program development of jiangsu higher education institutions
LinkOut - more resources
Full Text Sources

