Computational determination of toxicity risks associated with a selection of approved drugs having demonstrated activity against COVID-19
- PMID: 34674775
- PMCID: PMC8529228
- DOI: 10.1186/s40360-021-00519-5
Computational determination of toxicity risks associated with a selection of approved drugs having demonstrated activity against COVID-19
Abstract
Background: The emergence and rapid spread of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) in thelate 2019 has caused a devastating global pandemic of the severe pneumonia-like disease coronavirus disease 2019 (COVID-19). Although vaccines have been and are being developed, they are not accessible to everyone and not everyone can receive these vaccines. Also, it typically takes more than 10 years until a new therapeutic agent is approved for usage. Therefore, repurposing of known drugs can lend itself well as a key approach for significantly expediting the development of new therapies for COVID-19.
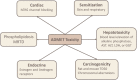
Methods: We have incorporated machine learning-based computational tools and in silico models into the drug discovery process to predict Adsorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) profiles of 90 potential drugs for COVID-19 treatment identified from two independent studies mainly with the purpose of mitigating late-phase failures because of inferior pharmacokinetics and toxicity.
Results: Here, we summarize the cardiotoxicity and general toxicity profiles of 90 potential drugs for COVID-19 treatment and outline the risks of repurposing and propose a stratification of patients accordingly. We shortlist a total of five compounds based on their non-toxic properties.
Conclusion: In summary, this manuscript aims to provide a potentially useful source of essential knowledge on toxicity assessment of 90 compounds for healthcare practitioners and researchers to find off-label alternatives for the treatment for COVID-19. The majority of the molecules discussed in this manuscript have already moved into clinical trials and thus their known pharmacological and human safety profiles are expected to facilitate a fast track preclinical and clinical assessment for treating COVID-19.
Keywords: COVID-19; Repurposing drugs, hERG); Toxicity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. Addendum: a pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;588(7836):E6. doi: 10.1038/s41586-020-2951-z. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous