Neuromedin B modulates phosphate-induced vascular calcification
- PMID: 34674793
- PMCID: PMC8633520
- DOI: 10.5483/BMBRep.2021.54.11.089
Neuromedin B modulates phosphate-induced vascular calcification
Abstract
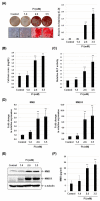
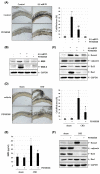
Vascular calcification is the heterotopic accumulation of calcium phosphate salts in the vascular tissue and is highly correlated with increased cardiovascular morbidity and mortality. In this study, we found that the expression of neuromedin B (NMB) and NMB receptor is upregulated in phosphate-induced calcification of vascular smooth muscle cells (VSMCs). Silencing of NMB or treatment with NMB receptor antagonist, PD168368, inhibited the phosphate-induced osteogenic differentiation of VSMCs by inhibiting Wnt/β-catenin signaling and VSMC apoptosis. PD168368 also attenuated the arterial calcification in cultured aortic rings and in a rat model of chronic kidney disease. The results of this study suggest that NMB-NMB receptor axis may have potential therapeutic value in the diagnosis and treatment of vascular calcification. [BMB Reports 2021; 54(11): 569-574].
Conflict of interest statement
The authors have no conflicting interests.
Figures


Similar articles
-
Overexpression of c1q/tumor necrosis factor-related protein-3 promotes phosphate-induced vascular smooth muscle cell calcification both in vivo and in vitro.Arterioscler Thromb Vasc Biol. 2014 May;34(5):1002-10. doi: 10.1161/ATVBAHA.114.303301. Epub 2014 Feb 27. Arterioscler Thromb Vasc Biol. 2014. PMID: 24578384
-
O-GlcNAc transferase promotes vascular smooth muscle calcification through modulating Wnt/β-catenin signaling.FASEB J. 2024 Dec 13;38(24):e70271. doi: 10.1096/fj.202401649RR. FASEB J. 2024. PMID: 39704274
-
Resveratrol ameliorates high-phosphate-induced VSMCs to osteoblast-like cells transdifferentiation and arterial medial calcification in CKD through regulating Wnt/β-catenin signaling.Eur J Pharmacol. 2022 Jun 15;925:174953. doi: 10.1016/j.ejphar.2022.174953. Epub 2022 Apr 26. Eur J Pharmacol. 2022. PMID: 35483665
-
Magnesium Counteracts Vascular Calcification: Passive Interference or Active Modulation?Arterioscler Thromb Vasc Biol. 2017 Aug;37(8):1431-1445. doi: 10.1161/ATVBAHA.117.309182. Epub 2017 Jun 29. Arterioscler Thromb Vasc Biol. 2017. PMID: 28663256 Review.
-
[The mechanisms of high-phosphate-induced vascular calcification in patients with chronic kidney disease].Sheng Li Ke Xue Jin Zhan. 2014 Feb;45(1):21-6. Sheng Li Ke Xue Jin Zhan. 2014. PMID: 24873139 Review. Chinese.
Cited by
-
Inhibition of bromodomain regulates cellular senescence in pancreatic adenocarcinoma.Int J Clin Exp Pathol. 2024 Oct 15;17(10):360-370. doi: 10.62347/BKNQ9812. eCollection 2024. Int J Clin Exp Pathol. 2024. PMID: 39544715 Free PMC article.
-
Bombesins: A New Frontier in Hybrid Compound Development.Pharmaceutics. 2023 Nov 7;15(11):2597. doi: 10.3390/pharmaceutics15112597. Pharmaceutics. 2023. PMID: 38004575 Free PMC article. Review.
-
Wnt/β-catenin signaling activator restores hair regeneration suppressed by diabetes mellitus.BMB Rep. 2022 Nov;55(11):559-564. doi: 10.5483/BMBRep.2022.55.11.081. BMB Rep. 2022. PMID: 36016500 Free PMC article.
-
Protective effects of cell permeable Tat-PIM2 protein on oxidative stress induced dopaminergic neuronal cell death.Heliyon. 2023 Apr 29;9(5):e15945. doi: 10.1016/j.heliyon.2023.e15945. eCollection 2023 May. Heliyon. 2023. PMID: 37223703 Free PMC article.
-
Differentially Expressed Genes and Molecular Susceptibility to Human Age-Related Diseases.Int J Mol Sci. 2023 Feb 16;24(4):3996. doi: 10.3390/ijms24043996. Int J Mol Sci. 2023. PMID: 36835409 Free PMC article.

