Mortality trends in pulmonary arterial hypertension in Canada: a temporal analysis of survival per ESC/ERS guideline era
- PMID: 34675044
- PMCID: PMC9160389
- DOI: 10.1183/13993003.01552-2021
Mortality trends in pulmonary arterial hypertension in Canada: a temporal analysis of survival per ESC/ERS guideline era
Abstract
Background: The evolution in pulmonary arterial hypertension (PAH) management has been summarised in three iterations of the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines. No study has assessed whether changes in management, as reflected in the changing guidelines, has translated to improved long-term survival in PAH.
Methods: We performed a mixed retrospective/prospective analysis of treatment-naïve, incident PAH patients (n=392) diagnosed at three major centres in Canada from 2009 to 2021. Patients were divided into two groups based on their diagnosis date and in accordance with the 2009 and 2015 ESC/ERS guideline iterations. Overall survival was assessed based on date of diagnosis and initial treatment strategy (i.e. monotherapy versus combination therapy).
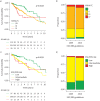
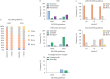
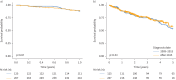
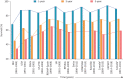
Results: There was a shift towards more aggressive upfront management with combination therapy in Canada after the publication of the 2015 ESC/ERS guidelines (10.4% and 30.8% in patients from 2009 to 2015 and 36.0% and 57.4% in patients diagnosed after 2015 for baseline and 2-year follow-up, respectively). A key factor associated with combination therapy after 2015 was higher pulmonary vascular resistance (p=0.009). The 1-, 3- and 5-year survival rates in Canada were 89.2%, 75.6% and 56.0%, respectively. Despite changes in management, there was no improvement in long-term survival before and after publication of the 2015 ESC/ERS guidelines (p=0.53).
Conclusions: There was an increase in the use of initial and sequential combination therapy in Canada after publication of the 2015 ESC/ERS guidelines, which was not associated with improved long-term survival. These data highlight the continued difficulties of managing this aggressive pulmonary disease in an era without a cure.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: J.G.E. Zelt has nothing to disclose. Conflict of interest: J. Sugarman has nothing to disclose. Conflict of interest: J. Weatherald reports grants, personal fees and nonfinancial support from Janssen Inc. and Actelion, personal fees and nonfinancial support from Bayer, personal fees from Novartis, outside the submitted work. Conflict of interest: A.C.R. Partridge has nothing to disclose. Conflict of interest: J. Liang has nothing to disclose. Conflict of interest: J. Swiston reports personal fees from Janssen J&J, outside the submitted work. Conflict of interest: N. Brunner reports personal fees from Janssen Pharmaceuticals, outside the submitted work. Conflict of interest: G. Chandy reports personal fees for advisory board work and consultancy from Bayer Pharmaceuticals, personal fees for advisory board work, lectures and consultancy from Janssen Pharmaceuticals, personal fees for advisory board work from Apotex, during the conduct of the study. Conflict of interest: D.J. Stewart reports other (equity) from Northern Therapeutics, outside the submitted work. Conflict of interest: V. Contreras-Dominguez has nothing to disclose. Conflict of interest: M. Thakrar reports grants from Reata Pharmaceuticals, outside the submitted work; and advisory board work for Janssen Pharmaceuticals. Conflict of interest: D. Helmersen reports grants from Gilead, Janssen and United Therapeutics, outside the submitted work. Conflict of interest: R. Varughese has nothing to disclose. Conflict of interest: N. Hirani reports grants (paid to institution) from Bayer, United Therapeutics and Northern Therapeutics, grants (paid to institution) and other (advisory board membership) from Actelion/Janssen, outside the submitted work. Conflict of interest: F. Umar has nothing to disclose. Conflict of interest: R. Dunne has nothing to disclose. Conflict of interest: C. Doyle-Cox has nothing to disclose. Conflict of interest: J. Foxall has nothing to disclose. Conflict of interest: L. Mielniczuk reports grants and personal fees for lectures from Janssen, grants from Bayer, outside the submitted work.
Figures

Comment in
-
To be or not to be… treated with initial combination therapy, that is the (PAH) question.Eur Respir J. 2022 Jun 2;59(6):2200390. doi: 10.1183/13993003.00390-2022. Print 2022 Jun. Eur Respir J. 2022. PMID: 35654453 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
