Soil chemistry determines whether defensive plant secondary metabolites promote or suppress herbivore growth
- PMID: 34675080
- PMCID: PMC8639379
- DOI: 10.1073/pnas.2109602118
Soil chemistry determines whether defensive plant secondary metabolites promote or suppress herbivore growth
Abstract
Plant secondary (or specialized) metabolites mediate important interactions in both the rhizosphere and the phyllosphere. If and how such compartmentalized functions interact to determine plant-environment interactions is not well understood. Here, we investigated how the dual role of maize benzoxazinoids as leaf defenses and root siderophores shapes the interaction between maize and a major global insect pest, the fall armyworm. We find that benzoxazinoids suppress fall armyworm growth when plants are grown in soils with very low available iron but enhance growth in soils with higher available iron. Manipulation experiments confirm that benzoxazinoids suppress herbivore growth under iron-deficient conditions and in the presence of chelated iron but enhance herbivore growth in the presence of free iron in the growth medium. This reversal of the protective effect of benzoxazinoids is not associated with major changes in plant primary metabolism. Plant defense activation is modulated by the interplay between soil iron and benzoxazinoids but does not explain fall armyworm performance. Instead, increased iron supply to the fall armyworm by benzoxazinoids in the presence of free iron enhances larval performance. This work identifies soil chemistry as a decisive factor for the impact of plant secondary metabolites on herbivore growth. It also demonstrates how the multifunctionality of plant secondary metabolites drives interactions between abiotic and biotic factors, with potential consequences for plant resistance in variable environments.
Keywords: benzoxazinoids; herbivore resistance; maize; plant herbivore interactions; plant secondary metabolites.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

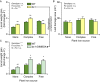
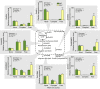

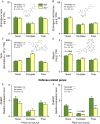
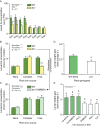
Similar articles
-
Plant iron acquisition strategy exploited by an insect herbivore.Science. 2018 Aug 17;361(6403):694-697. doi: 10.1126/science.aat4082. Epub 2018 Aug 16. Science. 2018. PMID: 30115808
-
Turnabout Is Fair Play: Herbivory-Induced Plant Chitinases Excreted in Fall Armyworm Frass Suppress Herbivore Defenses in Maize.Plant Physiol. 2016 May;171(1):694-706. doi: 10.1104/pp.15.01854. Epub 2016 Mar 15. Plant Physiol. 2016. PMID: 26979328 Free PMC article.
-
Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota.Nat Commun. 2018 Jul 16;9(1):2738. doi: 10.1038/s41467-018-05122-7. Nat Commun. 2018. PMID: 30013066 Free PMC article.
-
The Chemical Ecology of Benzoxazinoids.Chimia (Aarau). 2022 Nov 30;76(11):928-938. doi: 10.2533/chimia.2022.928. Chimia (Aarau). 2022. PMID: 38069788 Review.
-
Soil abiotic factors influence interactions between belowground herbivores and plant roots.J Exp Bot. 2013 Mar;64(5):1295-303. doi: 10.1093/jxb/ert007. J Exp Bot. 2013. PMID: 23505310 Review.
Cited by
-
BpWRKY6 regulates insect resistance by affecting jasmonic acid and terpenoid synthesis in Betula platyphylla.Plant Biotechnol J. 2025 Sep;23(9):3682-3696. doi: 10.1111/pbi.70169. Epub 2025 Jun 10. Plant Biotechnol J. 2025. PMID: 40493356 Free PMC article.
-
Plant growth and stress-regulating metabolite response to biochar utilization boost crop traits and soil health.Front Plant Sci. 2023 Oct 12;14:1271490. doi: 10.3389/fpls.2023.1271490. eCollection 2023. Front Plant Sci. 2023. PMID: 37900767 Free PMC article.
-
Beyond Photoprotection: The Multifarious Roles of Flavonoids in Plant Terrestrialization.Int J Mol Sci. 2022 May 9;23(9):5284. doi: 10.3390/ijms23095284. Int J Mol Sci. 2022. PMID: 35563675 Free PMC article. Review.
-
From trade-off to synergy: how nutrient status modulates plant resistance to herbivorous insects?Adv Biotechnol (Singap). 2024 Oct 8;2(4):37. doi: 10.1007/s44307-024-00045-5. Adv Biotechnol (Singap). 2024. PMID: 39883238 Free PMC article. Review.
-
Two leucine-rich repeat receptor-like kinases initiate herbivory defense responses in tea plants.Hortic Res. 2024 Oct 2;12(1):uhae281. doi: 10.1093/hr/uhae281. eCollection 2025 Jan. Hortic Res. 2024. PMID: 39850371 Free PMC article.
References
-
- Farina S. C., Kane E. A., Hernandez L. P., Multifunctional structures and multistructural functions: Integration in the evolution of biomechanical systems. Integr. Comp. Biol. 59, 338–345 (2019). - PubMed
-
- Tawfik D. S., Messy biology and the origins of evolutionary innovations. Nat. Chem. Biol. 6, 692–696 (2010). - PubMed
-
- Greenspan R. J., The flexible genome. Nat. Rev. Genet. 2, 383–387 (2001). - PubMed
-
- Neilson E. H., Goodger J. Q. D., Woodrow I. E., Møller B. L., Plant chemical defense: At what cost? Trends Plant Sci. 18, 250–258 (2013). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

