Novel Redirected T-Cell Immunotherapies for Advanced Prostate Cancer
- PMID: 34675084
- PMCID: PMC8866199
- DOI: 10.1158/1078-0432.CCR-21-1483
Novel Redirected T-Cell Immunotherapies for Advanced Prostate Cancer
Abstract
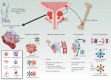
Immunotherapy has failed to achieve durable remissions in advanced prostate cancer patients. More potent T-cell-redirecting strategies may be needed to overcome the immunologically exclusive and suppressive tumor microenvironment. Clinical trials are underway, seeking to define the optimal target for T-cell redirection, such as PSMA, PSCA, or STEAP-1, as well as the optimal strategy, with CAR or bispecific antibodies. As results continue to emerge from these trials, understanding differential toxicity and efficacy of these therapies based on their targets and functional modifications will be key to advancing these promising therapies toward clinical practice. This review provides a unique depth and breadth of perspective regarding the diverse immunotherapy strategies currently under clinical investigation for men with advanced prostate cancer.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. - PubMed
-
- Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. . Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184–043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700–12. - PMC - PubMed
-
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. . Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–22. - PubMed
-
- Mehra N, Seed G, Lambros M, Sharp A, Fontes MS, Crespo M, et al. . Myeloid-derived suppressor cells (MDSCs) in metastatic castration-resistant prostate cancer (CRPC) patients (PTS). Ann Oncol 2016;27:vi257.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

