Intracellular delivery of protein drugs with an autonomously lysing bacterial system reduces tumor growth and metastases
- PMID: 34675204
- PMCID: PMC8531320
- DOI: 10.1038/s41467-021-26367-9
Intracellular delivery of protein drugs with an autonomously lysing bacterial system reduces tumor growth and metastases
Abstract
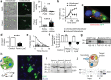
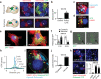
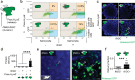
Critical cancer pathways often cannot be targeted because of limited efficiency crossing cell membranes. Here we report the development of a Salmonella-based intracellular delivery system to address this challenge. We engineer genetic circuits that (1) activate the regulator flhDC to drive invasion and (2) induce lysis to release proteins into tumor cells. Released protein drugs diffuse from Salmonella containing vacuoles into the cellular cytoplasm where they interact with their therapeutic targets. Control of invasion with flhDC increases delivery over 500 times. The autonomous triggering of lysis after invasion makes the platform self-limiting and prevents drug release in healthy organs. Bacterial delivery of constitutively active caspase-3 blocks the growth of hepatocellular carcinoma and lung metastases, and increases survival in mice. This success in targeted killing of cancer cells provides critical evidence that this approach will be applicable to a wide range of protein drugs for the treatment of solid tumors.
© 2021. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: V.R., N.V.D., and N.S.F. are founders of Ernest Pharmaceuticals, LLC. The remaining authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

