DNA damage response of haematopoietic stem and progenitor cells to high-LET neutron irradiation
- PMID: 34675263
- PMCID: PMC8531011
- DOI: 10.1038/s41598-021-00229-2
DNA damage response of haematopoietic stem and progenitor cells to high-LET neutron irradiation
Abstract
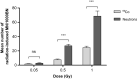
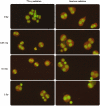
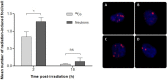
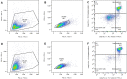
The radiosensitivity of haematopoietic stem and progenitor cells (HSPCs) to neutron radiation remains largely underexplored, notwithstanding their potential role as target cells for radiation-induced leukemogenesis. New insights are required for radiation protection purposes, particularly for aviation, space missions, nuclear accidents and even particle therapy. In this study, HSPCs (CD34+CD38+ cells) were isolated from umbilical cord blood and irradiated with 60Co γ-rays (photons) and high energy p(66)/Be(40) neutrons. At 2 h post-irradiation, a significantly higher number of 1.28 ± 0.12 γ-H2AX foci/cell was observed after 0.5 Gy neutrons compared to 0.84 ± 0.14 foci/cell for photons, but this decreased to similar levels for both radiation qualities after 18 h. However, a significant difference in late apoptosis was observed with Annexin-V+/PI+ assay between photon and neutron irradiation at 18 h, 43.17 ± 6.10% versus 55.55 ± 4.87%, respectively. A significant increase in MN frequency was observed after both 0.5 and 1 Gy neutron irradiation compared to photons illustrating higher levels of neutron-induced cytogenetic damage, while there was no difference in the nuclear division index between both radiation qualities. The results point towards a higher induction of DNA damage after neutron irradiation in HSPCs followed by error-prone DNA repair, which contributes to genomic instability and a higher risk of leukemogenesis.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- UNSCEAR . Sources, Effects and Risks of ionizing radiation. New York: United Nations Scientific Committee on the Effects of Atomic Radiation; 2013.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

