Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut
- PMID: 34675385
- PMCID: PMC8556157
- DOI: 10.1038/s41564-021-00970-4
Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut
Abstract
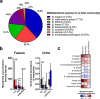
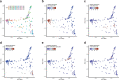
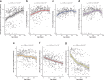
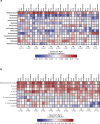
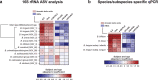
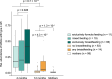
Breastfeeding profoundly shapes the infant gut microbiota, which is critical for early life immune development, and the gut microbiota can impact host physiology in various ways, such as through the production of metabolites. However, few breastmilk-dependent microbial metabolites mediating host-microbiota interactions are currently known. Here, we demonstrate that breastmilk-promoted Bifidobacterium species convert aromatic amino acids (tryptophan, phenylalanine and tyrosine) into their respective aromatic lactic acids (indolelactic acid, phenyllactic acid and 4-hydroxyphenyllactic acid) via a previously unrecognized aromatic lactate dehydrogenase (ALDH). The ability of Bifidobacterium species to convert aromatic amino acids to their lactic acid derivatives was confirmed using monocolonized mice. Longitudinal profiling of the faecal microbiota composition and metabolome of Danish infants (n = 25), from birth until 6 months of age, showed that faecal concentrations of aromatic lactic acids are correlated positively with the abundance of human milk oligosaccharide-degrading Bifidobacterium species containing the ALDH, including Bifidobacterium longum, B. breve and B. bifidum. We further demonstrate that faecal concentrations of Bifidobacterium-derived indolelactic acid are associated with the capacity of these samples to activate in vitro the aryl hydrocarbon receptor (AhR), a receptor important for controlling intestinal homoeostasis and immune responses. Finally, we show that indolelactic acid modulates ex vivo immune responses of human CD4+ T cells and monocytes in a dose-dependent manner by acting as an agonist of both the AhR and hydroxycarboxylic acid receptor 3 (HCA3). Our findings reveal that breastmilk-promoted Bifidobacterium species produce aromatic lactic acids in the gut of infants and suggest that these microbial metabolites may impact immune function in early life.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










Comment in
-
Breastfeeding promotes bifidobacterial immunomodulatory metabolites.Nat Microbiol. 2021 Nov;6(11):1335-1336. doi: 10.1038/s41564-021-00975-z. Nat Microbiol. 2021. PMID: 34675386 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

