Single-molecule mechanical fingerprinting with DNA nanoswitch calipers
- PMID: 34675411
- PMCID: PMC8678201
- DOI: 10.1038/s41565-021-00979-0
Single-molecule mechanical fingerprinting with DNA nanoswitch calipers
Abstract
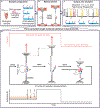
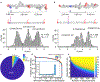
Decoding the identity of biomolecules from trace samples is a longstanding goal in the field of biotechnology. Advances in DNA analysis have substantially affected clinical practice and basic research, but corresponding developments for proteins face challenges due to their relative complexity and our inability to amplify them. Despite progress in methods such as mass spectrometry and mass cytometry, single-molecule protein identification remains a highly challenging objective. Towards this end, we combine DNA nanotechnology with single-molecule force spectroscopy to create a mechanically reconfigurable DNA nanoswitch caliper capable of measuring multiple coordinates on single biomolecules with atomic resolution. Using optical tweezers, we demonstrate absolute distance measurements with ångström-level precision for both DNA and peptides, and using multiplexed magnetic tweezers, we demonstrate quantification of relative abundance in mixed samples. Measuring distances between DNA-labelled residues, we perform single-molecule fingerprinting of synthetic and natural peptides, and show discrimination, within a heterogeneous population, between different posttranslational modifications. DNA nanoswitch calipers are a powerful and accessible tool for characterizing distances within nanoscale complexes that will enable new applications in fields such as single-molecule proteomics.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
W.M.S. and W.P.W. have filed patent applications for various aspects of this work.
Figures


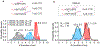

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

