The SARS-CoV-2 main protease Mpro causes microvascular brain pathology by cleaving NEMO in brain endothelial cells
- PMID: 34675436
- PMCID: PMC8553622
- DOI: 10.1038/s41593-021-00926-1
The SARS-CoV-2 main protease Mpro causes microvascular brain pathology by cleaving NEMO in brain endothelial cells
Abstract
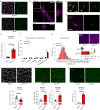
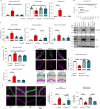
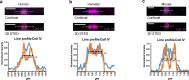
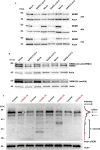
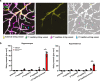
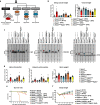
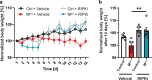
Coronavirus disease 2019 (COVID-19) can damage cerebral small vessels and cause neurological symptoms. Here we describe structural changes in cerebral small vessels of patients with COVID-19 and elucidate potential mechanisms underlying the vascular pathology. In brains of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected individuals and animal models, we found an increased number of empty basement membrane tubes, so-called string vessels representing remnants of lost capillaries. We obtained evidence that brain endothelial cells are infected and that the main protease of SARS-CoV-2 (Mpro) cleaves NEMO, the essential modulator of nuclear factor-κB. By ablating NEMO, Mpro induces the death of human brain endothelial cells and the occurrence of string vessels in mice. Deletion of receptor-interacting protein kinase (RIPK) 3, a mediator of regulated cell death, blocks the vessel rarefaction and disruption of the blood-brain barrier due to NEMO ablation. Importantly, a pharmacological inhibitor of RIPK signaling prevented the Mpro-induced microvascular pathology. Our data suggest RIPK as a potential therapeutic target to treat the neuropathology of COVID-19.
© 2021. The Author(s).
Conflict of interest statement
L.C. and R.V. are under paid employment by Hoffman La Roche. M. Zelic and D.O. are under paid employment by Sanofi. M.K. worked as a part-time consultant of Immungenetics AG. All other authors declare no competing interests.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

