Factors that Influence the Reported Sensitivity of Rapid Antigen Testing for SARS-CoV-2
- PMID: 34675892
- PMCID: PMC8524138
- DOI: 10.3389/fmicb.2021.714242
Factors that Influence the Reported Sensitivity of Rapid Antigen Testing for SARS-CoV-2
Abstract
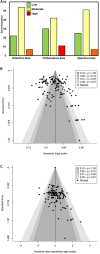
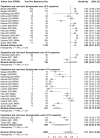
Tests that detect the presence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) antigen in clinical specimens from the upper respiratory tract can provide a rapid means of coronavirus disease 2019 (COVID-19) diagnosis and help identify individuals who may be infectious and should isolate to prevent SARS-CoV-2 transmission. This systematic review assesses the diagnostic accuracy of SARS-CoV-2 antigen detection in COVID-19 symptomatic and asymptomatic individuals compared to quantitative reverse transcription polymerase chain reaction (RT-qPCR) and summarizes antigen test sensitivity using meta-regression. In total, 83 studies were included that compared SARS-CoV-2 rapid antigen-based lateral flow testing (RALFT) to RT-qPCR for SARS-CoV-2. Generally, the quality of the evaluated studies was inconsistent; nevertheless, the overall sensitivity for RALFT was determined to be 75.0% (95% confidence interval: 71.0-78.0). Additionally, RALFT sensitivity was found to be higher for symptomatic vs. asymptomatic individuals and was higher for a symptomatic population within 7 days from symptom onset compared to a population with extended days of symptoms. Viral load was found to be the most important factor for determining SARS-CoV-2 antigen test sensitivity. Other design factors, such as specimen storage and anatomical collection type, also affect the performance of RALFT. RALFT and RT-qPCR testing both achieve high sensitivity when compared to SARS-CoV-2 viral culture.
Keywords: RT-PCR; SARS-CoV-2; diagnostic accuracy; meta-regression analysis; rapid antigen testing; systematic review and meta-analysis; test sensitivity; viral culture.
Copyright © 2021 Parvu, Gary, Mann, Lin, Mills, Cooper, Andrews, Manabe, Pekosz and Cooper.
Conflict of interest statement
VP, DG, Y-CL, DM, LC, JM, JA, and CC are employees of Becton, Dickinson and Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Abbott Diagnostics Scarborough Inc (2020a). BinaxNOWTM COVID-19 Ag CARD HOME TEST[package insert, EUA]. Scarborough, ME: Abbott Diagnostics Scarborough Inc.
-
- Abbott Diagnostics Scarborough Inc (2020b). BinaxNOWTM COVID-19 Ag CARD [package insert, EUA]. Scarborough, ME: Abbott Diagnostics Scarborough Inc.
-
- Abdulrahman A., Mustafa F., Alawadhi A. I., Alansari Q., Alalawi B., Alqahtani M. (2020). Comparison of SARS-COV-2 nasal antigen test to nasopharyngeal RT-PCR in mildly symptomatic patients. medRxiv [Preprint] 10.1101/2020.11.10.20228973 - DOI
-
- Access Bio (2020). CareStartTM COVID-19 Atigen-Rapid Diagnostic Test for the Detection of SARS-CoV-2 Antigen [package insert, EUA]. Somerset, NJ: Access Bio, Inc.
LinkOut - more resources
Full Text Sources
Miscellaneous

