Deep (Meta)genomics and (Meta)transcriptome Analyses of Fungal and Bacteria Consortia From Aircraft Tanks and Kerosene Identify Key Genes in Fuel and Tank Corrosion
- PMID: 34675897
- PMCID: PMC8525681
- DOI: 10.3389/fmicb.2021.722259
Deep (Meta)genomics and (Meta)transcriptome Analyses of Fungal and Bacteria Consortia From Aircraft Tanks and Kerosene Identify Key Genes in Fuel and Tank Corrosion
Abstract
Microbial contamination of fuels, associated with a wide variety of bacteria and fungi, leads to decreased product quality and can compromise equipment performance by biofouling or microbiologically influenced corrosion. Detection and quantification of microorganisms are critical in monitoring fuel systems for an early detection of microbial contaminations. To address these challenges, we have analyzed six metagenomes, one transcriptome, and more than 1,200 fluid and swab samples taken from fuel tanks or kerosene. Our deep metagenome sequencing and binning approaches in combination with RNA-seq data and qPCR methods implied a metabolic symbiosis between fungi and bacteria. The most abundant bacteria were affiliated with α-, β-, and γ-Proteobacteria and the filamentous fungi Amorphotheca. We identified a high number of genes, which are related to kerosene degradation and biofilm formation. Surprisingly, a large number of genes coded enzymes involved in polymer degradation and potential bio-corrosion processes. Thereby, the transcriptionally most active microorganisms were affiliated with the genera Methylobacteria, Pseudomonas, Kocuria, Amorpotheka, Aspergillus, Fusarium, and Penicillium. Many not yet cultured bacteria and fungi appeared to contribute to the biofilm transcriptional activities. The largest numbers of transcripts were observed for dehydrogenase, oxygenase, and exopolysaccharide production, attachment and pili/flagella-associated proteins, efflux pumps, and secretion systems as well as lipase and esterase activity.
Keywords: bacteria and fungi; biocorrosion; biofilm formation; biofouling; fuel contamination; kerosene and aircraft; omics analysis.
Copyright © 2021 Krohn, Bergmann, Qi, Indenbirken, Han, Perez-Garcia, Katzowitsch, Hägele, Lübcke, Siry, Riemann, Alawi and Streit.
Conflict of interest statement
BH, TL, CS, and RR were employed by company Lufthansa Technik AG HAM. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



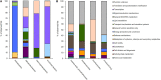

 high expression level,
high expression level,  low expression level.
low expression level.
References
-
- Andrews S. (2010). FASTQC. A quality control tool for high throughput sequence data. Available online at: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed October 21, 2019).
LinkOut - more resources
Full Text Sources

