Promoting Extracellular Electron Transfer of Shewanella oneidensis MR-1 by Optimizing the Periplasmic Cytochrome c Network
- PMID: 34675900
- PMCID: PMC8524038
- DOI: 10.3389/fmicb.2021.727709
Promoting Extracellular Electron Transfer of Shewanella oneidensis MR-1 by Optimizing the Periplasmic Cytochrome c Network
Abstract
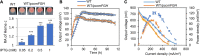
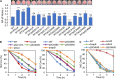
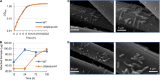
The low efficiency of extracellular electron transfer (EET) is a major bottleneck for Shewanella oneidensis MR-1 acting as an electroactive biocatalyst in bioelectrochemical systems. Although it is well established that a periplasmic c-type cytochrome (c-Cyt) network plays a critical role in regulating EET efficiency, the understanding of the network in terms of structure and electron transfer activity is obscure and partial. In this work, we attempted to systematically investigate the impacts of the network components on EET in their absence and overproduction individually in microbial fuel cell (MFC). We found that overexpression of c-Cyt CctA leads to accelerated electron transfer between CymA and the Mtr system, which function as the primary quinol oxidase and the outer-membrane (OM) electron hub in EET. In contrast, NapB, FccA, and TsdB in excess severely impaired EET, reducing EET capacity in MFC by more than 50%. Based on the results from both strategies, a series of engineered strains lacking FccA, NapB, and TsdB in combination while overproducing CctA were tested for a maximally optimized c-Cyt network. A strain depleted of all NapB, FccA, and TsdB with CctA overproduction achieved the highest maximum power density in MFCs (436.5 mW/m2), ∼3.62-fold higher than that of wild type (WT). By revealing that optimization of periplasmic c-Cyt composition is a practical strategy for improving EET efficiency, our work underscores the importance in understanding physiological and electrochemical characteristics of c-Cyts involved in EET.
Keywords: EET; MFC; Shewanella; cytochrome c; genetic engineering.
Copyright © 2021 Sun, Lin, Yu, Cheng and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Improvement of the electron transfer rate in Shewanella oneidensis MR-1 using a tailored periplasmic protein composition.Bioelectrochemistry. 2019 Oct;129:18-25. doi: 10.1016/j.bioelechem.2019.04.022. Epub 2019 Apr 30. Bioelectrochemistry. 2019. PMID: 31075535
-
Divergent Nrf Family Proteins and MtrCAB Homologs Facilitate Extracellular Electron Transfer in Aeromonas hydrophila.Appl Environ Microbiol. 2018 Nov 15;84(23):e02134-18. doi: 10.1128/AEM.02134-18. Print 2018 Dec 1. Appl Environ Microbiol. 2018. PMID: 30266730 Free PMC article.
-
A New Electron Shuttling Pathway Mediated by Lipophilic Phenoxazine via the Interaction with Periplasmic and Inner Membrane Proteins of Shewanella oneidensis MR-1.Environ Sci Technol. 2023 Feb 14;57(6):2636-2646. doi: 10.1021/acs.est.2c07862. Epub 2023 Jan 18. Environ Sci Technol. 2023. PMID: 36652548
-
Engineering S. oneidensis for Performance Improvement of Microbial Fuel Cell-a Mini Review.Appl Biochem Biotechnol. 2021 Apr;193(4):1170-1186. doi: 10.1007/s12010-020-03469-6. Epub 2020 Nov 17. Appl Biochem Biotechnol. 2021. PMID: 33200267 Review.
-
Cation-limited kinetic model for microbial extracellular electron transport via an outer membrane cytochrome C complex.Biophys Physicobiol. 2016 May 27;13:71-76. doi: 10.2142/biophysico.13.0_71. eCollection 2016. Biophys Physicobiol. 2016. PMID: 27924259 Free PMC article. Review.
Cited by
-
Engineering Shewanella carassii, a newly isolated exoelectrogen from activated sludge, to enhance methyl orange degradation and bioelectricity harvest.Synth Syst Biotechnol. 2022 May 1;7(3):918-927. doi: 10.1016/j.synbio.2022.04.010. eCollection 2022 Sep. Synth Syst Biotechnol. 2022. PMID: 35664929 Free PMC article.
-
NapB Restores cytochrome c biosynthesis in bacterial dsbD-deficient mutants.Commun Biol. 2022 Jan 21;5(1):87. doi: 10.1038/s42003-022-03034-3. Commun Biol. 2022. PMID: 35064202 Free PMC article.
-
Deciphering Houttuynia cordata extract as electron shuttles with anti-COVID-19 activity and its performance in microbial fuel cells.J Taiwan Inst Chem Eng. 2023 Apr;145:104838. doi: 10.1016/j.jtice.2023.104838. Epub 2023 Apr 3. J Taiwan Inst Chem Eng. 2023. PMID: 37051508 Free PMC article.
-
Living and Regenerative Material Encapsulating Self-Assembled Shewanella oneidensis-CdS Hybrids for Photocatalytic Biodegradation of Organic Dyes.Microorganisms. 2022 Dec 16;10(12):2501. doi: 10.3390/microorganisms10122501. Microorganisms. 2022. PMID: 36557754 Free PMC article.
-
Enhanced depolluting capabilities of microbial bioelectrochemical systems by synthetic biology.Synth Syst Biotechnol. 2023 May 25;8(3):341-348. doi: 10.1016/j.synbio.2023.05.005. eCollection 2023 Sep. Synth Syst Biotechnol. 2023. PMID: 37275577 Free PMC article.
References
-
- Borole A. P., Reguera G., Ringeisen B., Wang Z. W., Feng Y., Kim B. H. (2011). Electroactive biofilms: current status and future research needs. Energy Environ. Sci. 4 4813–4834. 10.1039/C1EE02511B - DOI
-
- Charania M. A., Brockman K. L., Zhang Y., Banerjee A., Pinchuk G. E., Fredrickson J. K., et al. (2009). Involvement of a membrane-bound class III adenylate cyclase in regulation of anaerobic respiration in Shewanella oneidensis MR-1. J. Bacteriol. 191 4298–4306. 10.1128/JB.01829-08 - DOI - PMC - PubMed
-
- Cheng Z. H., Xiong J. R., Min D., Cheng L., Liu D. F., Li W. W., et al. (2020). Promoting bidirectional extracellular electron transfer of Shewanella oneidensis MR-1 for hexavalent chromium reduction via elevating intracellular cAMP level. Biotechnol. Bioeng. 117 1294–1303. 10.1002/bit.27305 - DOI - PubMed
LinkOut - more resources
Full Text Sources

