Effectiveness of COVID-19 vaccines and their challenges (Review)
- PMID: 34676000
- PMCID: PMC8524740
- DOI: 10.3892/etm.2021.10843
Effectiveness of COVID-19 vaccines and their challenges (Review)
Abstract
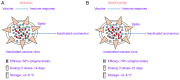
At the end of 2019, a new disease recognized such as severe acute respiratory syndrome (SARS), was reported in Wuhan, China. This disease was caused by an unknown SARS coronavirus 2 (SARS-CoV-2); a virus is characterized by high infectivity among humans. In some cases, this disease can be asymptomatic, while in other cases can induce flu-like symptoms or acute respiratory distress syndrome, pneumonia and death. For this reason, the World Health Organization and Public Health Emergency of International Concern declared a pandemic status in January 2020. Currently, numerous countries have been involved in the development of effective vaccines to protect humans against SARS-CoV-2 infection. The present review will discuss the four vaccines, AZD1222 (AstraZeneca or Vaxzevria), Janssen (Ad26.COV2.S), Moderna/mRNA-1273 and BioNTech/Fosun/Pfizer BNT162b1, that are currently in use worldwide to understand their efficacy, but also evaluate the difficulties and challenges of vaccine development. Although several questions should be addressed regarding these vaccines, the current review will examine the viral elements used in the coronavirus-19 vaccine that can play a crucial role in inducing a strong immune response, as well as the different adverse effects that they can cause to individuals.
Keywords: adverse effects; coronavirus-19; immunity; mRNA; pandemic; vaccines.
Copyright: © Marfe et al.
Figures


References
-
- World Health Organization (WHO): Novel Coronavirus (2019-nCoV). WHO, Geneva, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed February 7, 2020.
-
- World Health Organization (WHO): WHO Coronavirus (COVID-19) Dashboard. WHO, Geneva, 2021. https://covid19.who.int/. Accessed August 18, 2021.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
