ATR inhibition reverses the resistance of homologous recombination deficient MGMTlow/MMRproficient cancer cells to temozolomide
- PMID: 34676045
- PMCID: PMC8522839
- DOI: 10.18632/oncotarget.28090
ATR inhibition reverses the resistance of homologous recombination deficient MGMTlow/MMRproficient cancer cells to temozolomide
Abstract
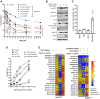
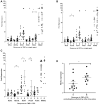
The therapeutic efficacy of temozolomide (TMZ) is hindered by inherent and acquired resistance. Biomarkers such as MGMT expression and MMR proficiency are used as predictors of response. However, not all MGMTlow/-ve/MMRproficient patients benefit from TMZ treatment, indicating a need for additional patient selection criteria. We explored the role of ATR in mediating TMZ resistance and whether ATR inhibitors (ATRi) could reverse this resistance in multiple cancer lines. We observed that only 31% of MGMTlow/-ve/MMRproficient patient-derived and established cancer lines are sensitive to TMZ at clinically relevant concentrations. TMZ treatment resulted in DNA damage signaling in both sensitive and resistant lines, but prolonged G2/M arrest and cell death were exclusive to sensitive models. Inhibition of ATR but not ATM, sensitized the majority of resistant models to TMZ and resulted in measurable DNA damage and persistent growth inhibition. Also, compromised homologous recombination (HR) via RAD51 or BRCA1 loss only conferred sensitivity to TMZ when combined with an ATRi. Furthermore, low REV3L mRNA expression correlated with sensitivity to the TMZ and ATRi combination in vitro and in vivo. This suggests that HR defects and low REV3L levels could be useful selection criteria for enhanced clinical efficacy of an ATRi plus TMZ combination.
Keywords: ATR; HR; MMR; REV3L; TMZ.
Copyright: © 2021 El Touny et al.
Conflict of interest statement
CONFLICTS OF INTEREST Authors have no conflicts of interest to declare.
Figures




References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, et al., and European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, and National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–96. 10.1056/NEJMoa043330. - DOI - PubMed
-
- Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G, Lorigan P, Kendra KL, Maio M, et al.. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013; 31:616–22. 10.1200/JCO.2012.44.6112. - DOI - PMC - PubMed
-
- Knizhnik AV, Roos WP, Nikolova T, Quiros S, Tomaszowski KH, Christmann M, Kaina B. Survival and death strategies in glioma cells: autophagy, senescence and apoptosis triggered by a single type of temozolomide-induced DNA damage. PLoS One. 2013; 8:e55665. 10.1371/journal.pone.0055665. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

