Study on the Effect of Combination of Prednisone and Vitamin D in the Treatment of Primary Nephrotic Syndrome in Children
- PMID: 34676063
- PMCID: PMC8526256
- DOI: 10.1155/2021/7932721
Study on the Effect of Combination of Prednisone and Vitamin D in the Treatment of Primary Nephrotic Syndrome in Children
Retraction in
-
Retracted: Study on the Effect of Combination of Prednisone and Vitamin D in the Treatment of Primary Nephrotic Syndrome in Children.J Healthc Eng. 2023 Jan 17;2023:9784704. doi: 10.1155/2023/9784704. eCollection 2023. J Healthc Eng. 2023. PMID: 36704574 Free PMC article.
Abstract
Objective: To study the effect of prednisone combined with vitamin D in the treatment of primary nephrotic syndrome in children.
Method: 73 cases of primary nephrotic syndrome admitted to the nephrology department of our hospital were randomly selected and retrospectively analyzed. 36 cases were treated with prednisone as the control group, and 37 cases were treated with prednisone combined with vitamin D as the observation group. The efficacy was compared after 3 months of continuous treatment.
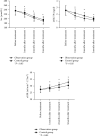
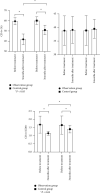
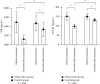
Result: After 3 months of treatment, the blood calcium of the observation group was higher than that of the control group, PTH was lower than that of the control group, and 25-(OH)2D3 and 1,25-(OH)2D3 were higher than those of the control group (P < 0.05). After 1, 2, and 3 months of treatment in the observation group, Scr and 24-h urine protein quantification were lower than those in the control group and eGFR was higher than that in the control group (P < 0.05). CD4+ and CD4+/CD8+ were lower in the observation group than in the control group after 3 months of treatment (P < 0.05). The serum sTfR and TGF-β1 levels were lower in the observation group than in the control group after 3 months of treatment (P < 0.05). The total effective rate of the observation group was 83.78% after 3 months of combined treatment with prednisone and vitamin D, which was significantly higher than the total effective rate of the control group of 61.11% (P < 0.05). The incidence of nausea and vomiting, heartburn, headache, dry cough, hypercalcemia, and constipation during treatment in the observation group was not statistically different from that in the control group (P > 0.05).
Conclusion: Combined treatment of primary nephrotic syndrome in children with prednisone and vitamin D can more significantly improve the level of clinical indicators, improve renal function and immune function, and obtain more satisfactory efficacy, without significantly affecting the safety of treatment.
Copyright © 2021 Guoqun Zhou and Xiangdong Kong.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Tapia C., Bashir K. StatPearls [Internet] Treasure Island, FL, USA: StatPearls Publishing; 2020. Nephrotic Syndrome. - PubMed
-
- Zhang Y, Wang Y. Y., Fu R, Yan X. Evaluation of glucocorticoid treatment on different pathological types of primary nephrotic syndrome. Journal of biological regulators and homeostatic agents . 2019;33(2):427–432. - PubMed
-
- Aurelle M., Basmaison O., Ranchin B., et al. Intermittent cholecalciferol supplementation in children and teenagers followed in pediatric nephrology: data from a prospective single-center single-arm open trial. European Journal of Pediatrics . 2020;179(4):661–669. doi: 10.1007/s00431-019-03553-y. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

