Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19
- PMID: 34676087
- PMCID: PMC7313782
- DOI: 10.1093/nsr/nwaa113
Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19
Abstract
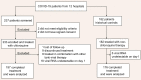
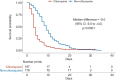
Effective therapies are urgently needed for the SARS-CoV-2 pandemic. Chloroquine has been proved to have antiviral effect against coronavirus in vitro. In this study, we aimed to assess the efficacy and safety of chloroquine with different doses in COVID-19. In this multicenter prospective observational study, we enrolled patients older than 18 years old with confirmed SARS-CoV-2 infection excluding critical cases from 12 hospitals in Guangdong and Hubei Provinces. Eligible patients received chloroquine phosphate 500 mg, orally, once (half dose) or twice (full dose) daily. Patients treated with non-chloroquine therapy were included as historical controls. The primary endpoint is the time to undetectable viral RNA. Secondary outcomes include the proportion of patients with undetectable viral RNA by day 10 and 14, hospitalization time, duration of fever, and adverse events. A total of 197 patients completed chloroquine treatment, and 176 patients were included as historical controls. The median time to achieve an undetectable viral RNA was shorter in chloroquine than in non-chloroquine (absolute difference in medians -6.0 days; 95% CI -6.0 to -4.0). The duration of fever is shorter in chloroquine (geometric mean ratio 0.6; 95% CI 0.5 to 0.8). No serious adverse events were observed in the chloroquine group. Patients treated with half dose experienced lower rate of adverse events than with full dose. Although randomized trials are needed for further evaluation, this study provides evidence for safety and efficacy of chloroquine in COVID-19 and suggests that chloroquine can be a cost-effective therapy for combating the COVID-19 pandemic.
Keywords: COVID-19; SARS-CoV-2; chloroquine; treatment.
© The Author(s) 2020. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
