Replication, pathogenicity, and transmission of SARS-CoV-2 in minks
- PMID: 34676095
- PMCID: PMC7798852
- DOI: 10.1093/nsr/nwaa291
Replication, pathogenicity, and transmission of SARS-CoV-2 in minks
Abstract
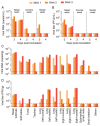
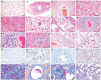
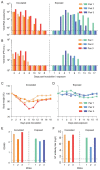
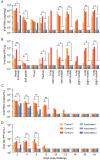
Minks are raised in many countries and have transmitted severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to humans. However, the biologic properties of SARS-CoV-2 in minks are largely unknown. Here, we investigated and found that SARS-CoV-2 replicates efficiently in both the upper and lower respiratory tracts, and transmits efficiently in minks via respiratory droplets; pulmonary lesions caused by SARS-CoV-2 in minks are similar to those seen in humans with COVID-19. We further found that a spike protein-based subunit vaccine largely prevented SARS-CoV-2 replication and lung damage caused by SARS-CoV-2 infection in minks. Our study indicates that minks are a useful animal model for evaluating the efficacy of drugs or vaccines against COVID-19 and that vaccination is a potential strategy to prevent minks from transmitting SARS-CoV-2.
Keywords: SARS-CoV-2; animal model; mink; pathogenicity; replication; transmission.
© The Author(s) 2020. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous
