Endostar (rh-endostatin) improves efficacy of concurrent chemoradiotherapy for locally advanced non-small cell lung cancer: A systematic review and meta-analysis
- PMID: 34676669
- PMCID: PMC8636201
- DOI: 10.1111/1759-7714.14188
Endostar (rh-endostatin) improves efficacy of concurrent chemoradiotherapy for locally advanced non-small cell lung cancer: A systematic review and meta-analysis
Abstract
Background: We aimed to clarify the benefits of the addition of rh-endostatin into concurrent chemoradiotherapy (CCRT) versus CCRT alone for locally advanced non-small cell lung cancer (NSCLC) by a meta-analysis.
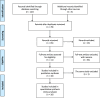
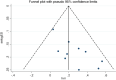
Methods: PubMed, Embase, Cochrane Central Register of Controlled Trials, Wanfang and Chinese National Knowledge Infrastructure (CNKI) were systematically screened from inception to November 2020 using the prespecified terms. Prospective trials (evaluating or) comparing the efficacy of endostar combined with CCRT and CCRT for locally advanced NSCLC were included. The primary endpoints were risk ratios (RRs) for objective response rate (ORR) and disease control rate (DCR). The secondary endpoints were RRs for overall survival (OS) and adverse events (AEs).
Results: Ten studies with 716 patients were included in this meta-analysis. Endostar combined with CCRT significantly improved ORR and DCR compared with CCRT. The RRs of ORR and DCR for endostar combined with CCRT versus CCRT were 1.263 (95% CI: 1.137-1.403, p < 0.001) and 1.274 (95% CI: 1.124-1.444, p < 0.001), respectively. Endostar combined with CCRT significantly improved one-year survival rate compared with CCRT with pooled RR = 1.113 (95% CI: 1.006-1.231, p = 0.038). Endostar combination treatments had similar incidences of main adverse events compared with CCRT (p > 0.05).
Conclusion: Endostar combined with CCRT is associated with significantly higher ORR, DCR and survival rate than CCRT with similar incidences of main adverse events in NSCLC.
Keywords: NSCLC; chemoradiotherapy; endostar; meta-analysis.
© 2021 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
Figures

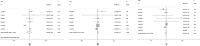
References
-
- Hanahan D, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. - PubMed
-
- Ge W, Cao DD, Wang HM, Jie FF, Zheng YF, Chen Y. Endostar combined with chemotherapy versus chemotherapy alone for advanced NSCLCs: a meta‐analysis. Asian Pac J Cancer Prev. 2011;12:2705–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

