Soft Hydrogel Inspired by Elastomeric Proteins
- PMID: 34676744
- PMCID: PMC8579378
- DOI: 10.1021/acsbiomaterials.1c00817
Soft Hydrogel Inspired by Elastomeric Proteins
Abstract
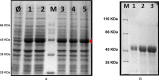
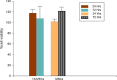
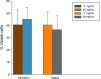
Elastin polypeptides based on -VPGVG- repeated motifs are widely used in the production of biomaterials because they are stimuli-responsive systems. On the other hand, glycine-rich sequences, mainly present in tropoelastin terminal domains, are responsible for the elastin self-assembly. In a previous study, we have recombinantly expressed a chimeric polypeptide, named resilin, elastin, and collagen (REC), inspired by glycine-rich motifs of elastin and containing resilin and collagen sequences as well. Herein, a three-block polypeptide, named (REC)3, was expressed starting from the previous monomer gene by introducing key modifications in the sequence. The choice was mandatory because the uneven distribution of the cross-linking sites in the monomer precluded the hydrogel production. In this work, the cross-linked polypeptide appeared as a soft hydrogel, as assessed by rheology, and the linear un-cross-linked trimer self-aggregated more rapidly than the REC monomer. The absence of cell-adhesive sequences did not affect cell viability, while it was functional to the production of a material presenting antiadhesive properties useful in the integration of synthetic devices in the body and preventing the invasion of cells.
Keywords: antiadhesive materials; circular dichroism; cytocompatibility; elastin; hydrogel.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

