All‑trans retinoic acid promotes macrophage phagocytosis and decreases inflammation via inhibiting CD14/TLR4 in acute lung injury
- PMID: 34676874
- PMCID: PMC8554390
- DOI: 10.3892/mmr.2021.12508
All‑trans retinoic acid promotes macrophage phagocytosis and decreases inflammation via inhibiting CD14/TLR4 in acute lung injury
Abstract
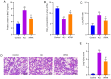
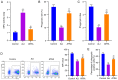
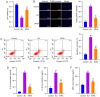
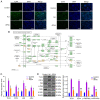
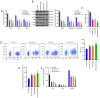
Acute lung injury (ALI) is a common clinical emergency and all‑trans retinoic acid (ATRA) can alleviate organ injury. Therefore, the present study investigated the role of ATRA in ALI. Lipopolysaccharide (LPS)‑induced ALI rats were treated with ATRA and the arterial partial pressure of oxygen (PaO2), lung wet/dry weight (W/D) ratio and protein content in the bronchial alveolar lavage fluid (BALF) were measured to evaluate the effect of ATRA on ALI rats. Alveolar macrophages were isolated from the BALF. The phagocytic function of macrophages was detected using the chicken erythrocyte phagocytosis method and flow cytometry. The viability of macrophages was measured using a Cell Counting Kit‑8 assay, and apoptosis was analyzed using a TUNEL assay and flow cytometry. The expression levels of Toll‑like receptor 4 (TLR4) and cluster of differentiation (CD)14 on the macrophage membrane were detected by immunofluorescence staining. The protein levels of TLR4, CD14, phosphorylated (p)‑65, p65, p‑IκBα and IκBα were analyzed using western blotting. The concentrations of IL‑6, IL‑1β and macrophage inflammatory protein‑2 in the plasma of rats were detected by ELISA. Macrophages were treated with IAXO‑102 (TLR4 inhibitor) to verify the involvement of CD14/TLR4 in the effect of ATRA on ALI. ATRA provided protection against LPS‑induced ALI, as evidenced by the increased PaO2 and reduced lung W/D ratio and protein content in the BALF. ATRA enhanced macrophage phagocytosis and viability and reduced apoptosis and inflammation in ALI rats. Mechanically, ATRA inhibited CD14 and TLR4 expression and NF‑κB pathway activation. ATRA enhanced macrophage phagocytosis and reduced inflammation by inhibiting the CD14/TLR4‑NF‑κB pathway in LPS‑induced ALI. In summary, ATRA inactivated the NF‑κB pathway by inhibiting the expression of CD14/TLR4 receptor in the alveolar macrophages of rats, thus enhancing the phagocytic function of macrophages in ALI rats, improving the activity of macrophages, inhibiting apoptosis, reducing the levels of inflammatory factors, and consequently playing a protective role in ALI model rats. This study may offer novel insights for the clinical management of ALI.
Keywords: Toll‑like receptor 4; acute lung injury; all‑trans retinoic acid; cluster of differentiation 14; inflammation; macrophage; phagocytosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
MicroRNA‑93 contributes to the suppression of lung inflammatory responses in LPS‑induced acute lung injury in mice via the TLR4/MyD88/NF‑κB signaling pathway.Int J Mol Med. 2020 Aug;46(2):561-570. doi: 10.3892/ijmm.2020.4610. Epub 2020 May 19. Int J Mol Med. 2020. PMID: 32468034 Free PMC article.
-
Yemazhui () ameliorates lipopolysaccharide-induced acute lung injury modulation of the toll-like receptor 4/nuclear factor kappa-B/nod-like receptor family pyrin domain-containing 3 protein signaling pathway and intestinal flora in rats.J Tradit Chin Med. 2024 Apr;44(2):303-314. doi: 10.19852/j.cnki.jtcm.20230510.001. J Tradit Chin Med. 2024. PMID: 38504536 Free PMC article.
-
Bakuchiol regulates TLR4/MyD88/NF-κB and Keap1/Nrf2/HO-1 pathways to protect against LPS-induced acute lung injury in vitro and in vivo.Naunyn Schmiedebergs Arch Pharmacol. 2024 May;397(5):3301-3312. doi: 10.1007/s00210-023-02813-x. Epub 2023 Nov 6. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37930390
-
[CD14 protein as a modulator of the inflammatory response].Postepy Biochem. 2024 Jan 30;69(4):274-282. doi: 10.18388/pb.2021_501. Print 2024 Jan 30. Postepy Biochem. 2024. PMID: 39012698 Review. Polish.
-
Role of CD14 in human disease.Immunology. 2023 Jul;169(3):260-270. doi: 10.1111/imm.13634. Epub 2023 Mar 27. Immunology. 2023. PMID: 36840585 Free PMC article. Review.
Cited by
-
Coelomocytes of the Oligochaeta earthworm Lumbricus terrestris (Linnaeus, 1758) as evolutionary key of defense: a morphological study.Zoological Lett. 2023 Mar 4;9(1):5. doi: 10.1186/s40851-023-00203-y. Zoological Lett. 2023. PMID: 36871038 Free PMC article.
-
Therapeutic Effects of Retinoic Acid in Lipopolysaccharide-Induced Cardiac Dysfunction: Network Pharmacology and Experimental Validation.J Inflamm Res. 2022 Aug 30;15:4963-4979. doi: 10.2147/JIR.S358374. eCollection 2022. J Inflamm Res. 2022. PMID: 36105385 Free PMC article.
-
Efficacy of terpenoids in attenuating pulmonary edema in acute lung injury: A meta-analysis of animal studies.Front Pharmacol. 2022 Aug 10;13:946554. doi: 10.3389/fphar.2022.946554. eCollection 2022. Front Pharmacol. 2022. PMID: 36034851 Free PMC article.
-
Immunomodulatory Microparticles Epigenetically Modulate T Cells and Systemically Ameliorate Autoimmune Arthritis.Adv Sci (Weinh). 2023 Apr;10(11):e2202720. doi: 10.1002/advs.202202720. Epub 2023 Mar 8. Adv Sci (Weinh). 2023. PMID: 36890657 Free PMC article.
-
β-Carotene enhances the expression of inflammation-related genes and histone H3 K9 acetylation, K4 dimethylation, and K36 trimethylation around these genes in juvenile macrophage-like THP-1 cells.Biochem Biophys Rep. 2022 Aug 15;31:101325. doi: 10.1016/j.bbrep.2022.101325. eCollection 2022 Sep. Biochem Biophys Rep. 2022. PMID: 35990579 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

