Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice
- PMID: 34676876
- PMCID: PMC8547541
- DOI: 10.3892/ijmm.2021.5052
Semaglutide attenuates seizure severity and ameliorates cognitive dysfunction by blocking the NLR family pyrin domain containing 3 inflammasome in pentylenetetrazole‑kindled mice
Abstract
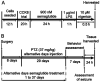
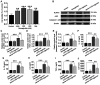
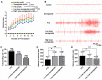
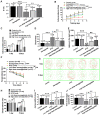
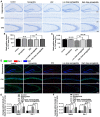
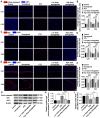
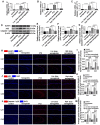
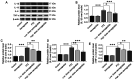
Epilepsy comorbidities and anti‑epileptic drugs (AEDs) are currently the main limitations of epilepsy treatment. Semaglutide is a glucagon like peptide‑1 analogue that has entered the market as a new once‑weekly drug for type II diabetes. The aim of the present study was to investigate the functions of semaglutide in epilepsy and inflammation models, in order to investigate its potential mechanism. In vitro, an inflammation model was established using lipopolysaccharide (LPS) and nigericin stimulation in BV2 cells. In vivo, chronic epilepsy model mice were generated using a pentylenetetrazole (PTZ) kindling method. BV2 cell proliferation was assessed using the Cell Counting Kit‑8. The effects of semaglutide on NLR family pyrin domain containing 3 (NLRP3) inflammasome activation and inflammatory cytokine secretion were determined using western blotting (WB) and ELISA. A lactate dehydrogenase (LDH) assay kit was used to detect the effect of semaglutide on LDH release. Electrocorticography and the modified Racine scale were used to assess seizure severity. Cognitive function was evaluated with behavioral assessment. Morphological changes in the hippocampus were observed with Nissl staining. Double immunofluorescence staining for NeuN and Iba‑1, WB and immunofluorescence analysis of apoptosis‑related proteins were used to evaluate neuronal apoptosis. The NLRP3 inflammasome was assessed by reverse transcription‑quantitative PCR, WB and immunofluorescence staining, and inflammatory cytokine release was evaluated by WB analysis in the hippocampus of C57/BL6J model mouse. Semaglutide attenuated the LPS‑ and nigericin‑induced inflammatory response and LDH release by blocking NLRP3 inflammasome activation in BV2 cells. Moreover, semaglutide decreased seizure severity, alleviated hippocampal neuronal apoptosis, ameliorated cognitive dysfunction, blocked NLRP3 inflammasome activation and decreased inflammatory cytokine secretion in PTZ‑kindled mice. These results indicated that semaglutide reduced seizure severity, exerted neuroprotective effects and ameliorated cognitive dysfunction, possibly via inhibition of NLRP3 inflammasome activation and inflammatory cytokine secretion. Semaglutide may therefore be a novel, promising adjuvant therapeutic for epilepsy and its associated comorbidities.
Keywords: NLR family pyrin domain containing 3 inflammasome; cognitive dysfunction; epilepsy; glucagon like peptide‑1; neuroprotection; semaglutide.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Oridonin exerts anticonvulsant profile and neuroprotective activity in epileptic mice by inhibiting NLRP3-mediated pyroptosis.Int Immunopharmacol. 2024 Jun 15;134:112247. doi: 10.1016/j.intimp.2024.112247. Epub 2024 May 16. Int Immunopharmacol. 2024. PMID: 38759374
-
Irisin ameliorates cognitive impairment in a lipopolysaccharide-induced neuroinflammation mouse model by inhibiting the NLRP3 inflammasome pathway in microglia.Neuropharmacology. 2025 Nov 1;278:110572. doi: 10.1016/j.neuropharm.2025.110572. Epub 2025 Jun 23. Neuropharmacology. 2025. PMID: 40562230
-
Mitigating seizure-induced cognitive deficits in mice induced with pentylenetetrazol by roflumilast through targeting the NLRP3 inflammasome/BDNF/SIRT3 pathway and regulating ferroptosis.Life Sci. 2025 Apr 1;366-367:123488. doi: 10.1016/j.lfs.2025.123488. Epub 2025 Feb 19. Life Sci. 2025. PMID: 39983820
-
Mechanism of NLRP3 Inflammasome in Epilepsy and Related Therapeutic Agents.Neuroscience. 2024 May 14;546:157-177. doi: 10.1016/j.neuroscience.2024.03.029. Epub 2024 Apr 3. Neuroscience. 2024. PMID: 38574797 Review.
-
The Role of Histone Deacetylases in NLRP3 Inflammasomesmediated Epilepsy.Curr Mol Med. 2024;24(8):980-1003. doi: 10.2174/1566524023666230731095431. Curr Mol Med. 2024. PMID: 37519210 Review.
Cited by
-
Muscone ameliorates myocardial ischemia‒reperfusion injury by promoting myocardial glycolysis.Heliyon. 2023 Nov 10;9(11):e22154. doi: 10.1016/j.heliyon.2023.e22154. eCollection 2023 Nov. Heliyon. 2023. PMID: 38045159 Free PMC article.
-
Targeting PTGS2/NF-κB Pathway: MG-132's Role in Reducing Ischemic Stroke Injury.Biochem Genet. 2025 Jun 7. doi: 10.1007/s10528-025-11155-7. Online ahead of print. Biochem Genet. 2025. PMID: 40481933
-
Astragalus polysaccharides ameliorate epileptogenesis, cognitive impairment, and neuroinflammation in a pentylenetetrazole-induced kindling mouse model.Front Pharmacol. 2024 Feb 9;15:1336122. doi: 10.3389/fphar.2024.1336122. eCollection 2024. Front Pharmacol. 2024. PMID: 38405667 Free PMC article.
-
Identification of a circRNA-mediated immune-related ceRNA network and circRNAs as diagnostic biomarkers in acute ischemic stroke.Eur J Med Res. 2025 Feb 18;30(1):114. doi: 10.1186/s40001-025-02356-2. Eur J Med Res. 2025. PMID: 39966952 Free PMC article.
-
Phosphorylated proteomics-based analysis of the effects of semaglutide on hippocampi of high-fat diet-induced-obese mice.Diabetol Metab Syndr. 2023 Mar 30;15(1):63. doi: 10.1186/s13098-023-01023-y. Diabetol Metab Syndr. 2023. PMID: 36998046 Free PMC article.
References
-
- González-H G, Contreras-García IJ, Sánchez-Huerta K, Queiroz CM, Gallardo Gudiño LR, Mendoza-Torreblanca JG, Zamudio SR. Levetiracetam reduced the basal excitability of the dentate gyrus without restoring impaired synaptic plasticity in rats with temporal lobe epilepsy. Brain Sci. 2020;10:E634. doi: 10.3390/brainsci10090634. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

