Chemogenetic stimulation of the Gi pathway in astrocytes suppresses neuroinflammation
- PMID: 34676988
- PMCID: PMC8532135
- DOI: 10.1002/prp2.822
Chemogenetic stimulation of the Gi pathway in astrocytes suppresses neuroinflammation
Abstract
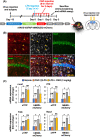
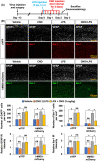
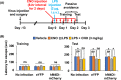
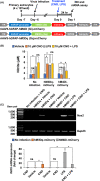
Engineered G protein-coupled receptors (GPCRs) are commonly used in chemogenetics as designer receptors exclusively activated by designer drugs (DREADDs). Although several GPCRs have been studied in astrocytes using a chemogenetic approach, the functional role of the astrocytic Gi pathway is not clear, as the literature is conflicting depending on the brain regions or behaviors investigated. In this study, we evaluated the role of the astrocytic Gi pathway in neuroinflammation using a Gi -coupled DREADD (hM4Di). Gi -DREADD was expressed in hippocampal astrocytes of a lipopolysaccharide (LPS)-induced neuroinflammation mouse model using adeno-associated viruses. We found that astrocyte Gi -DREADD stimulation using clozapine N-oxide (CNO) inhibits neuroinflammation, as characterized by decreased levels of proinflammatory cytokines, glial activation, and cognitive impairment in mice. Subsequent experiments using primary astrocyte cultures revealed that Gi -DREADD stimulation significantly downregulated LPS-induced expression of Nos2 mRNA and nitric oxide production. Similarly, in vitro calcium imaging showed that activation of the astrocytic Gi pathway attenuated intracellular calcium transients triggered by LPS treatment, suggesting a positive correlation between enhanced calcium transients and the inflammatory phenotype of astrocytes observed in the inflamed brain. Taken together, our results indicate that the astrocytic Gi pathway plays an inhibitory role in neuroinflammation, providing an opportunity to identify potential cellular and molecular targets to control neuroinflammation.
Keywords: Gi-DREADD; astrocyte; chemogenetics; hM4Di; neuroinflammation.
© 2021 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest in regards to this manuscript.
Figures
References
-
- Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol. 2015;55:399‐417. - PubMed
-
- Hirbec H, Déglon N, Foo LC, et al. Emerging technologies to study glial cells. Glia. 2020;68:1692‐1728. - PubMed
-
- Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging. 2008;29:1754‐1762. - PubMed
-
- Nam MH, Han KS, Lee J, et al. Activation of astrocytic mu‐opioid receptor causes conditioned place preference. Cell Rep. 2019;28:1154‐1166 e5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

