Trends in patient-reported outcome use in early phase dose-finding oncology trials - an analysis of ClinicalTrials.gov
- PMID: 34676991
- PMCID: PMC8607259
- DOI: 10.1002/cam4.4307
Trends in patient-reported outcome use in early phase dose-finding oncology trials - an analysis of ClinicalTrials.gov
Abstract
Background: Patient-reported adverse events (AEs) may be a useful adjunct to clinician-assessed AEs for assessing tolerability in early phase, dose-finding oncology trials (DFOTs). We reviewed DFOTs on ClinicalTrials.gov to describe trends in patient-reported outcome (PRO) use.
Methods: DFOTs commencing 01 January 2007 - 20 January 2020 with 'PROs' or 'quality of life' as an outcome were extracted and inclusion criteria confirmed. Study and PRO characteristics were extracted. Completed trials that reported PRO outcomes and published manuscripts on ClinicalTrials.gov were identified, and PRO reporting details were extracted.
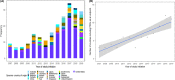
Results: 5.3% (548/10 372) DFOTs included PROs as an outcome. 231 (42.2%) were eligible: adult (224, 97%), solid tumour (175, 75.8%), and seamless phase 1/2 (108, 46.8%). PRO endpoints were identified in more trials (2.3 increase/year, 95% CI: 1.6-2.9) from an increasing variety of countries (0.7/year) (95% CI: 0.4-0.9) over time. PROs were typically secondary endpoints (207, 89.6%). 15/77 (19.5%) completed trials reported results on the ClinicalTrials.gov results database, and of those eight included their PRO results. Eighteen trials had published manuscripts available on ClinicalTrials.gov. Three (16.7%) used PROs to confirm the maximum tolerated dose. No trials identified who completed the PROs or how PROs were collected.
Conclusions: PRO use in DFOT has increased but remains limited. Future work should explore the role of PROs in DFOT and determine what guidelines are needed to standardise PRO use.
Keywords: clinical trials; drug development; oncology; patient-reported outcomes; phase 1; quality of life.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
AM has served on advisory boards and received fees from Merck, FARON, Novartis, Bayer and Janssen, which are all unrelated to this work. CY has received fees from FARON and honorarium from Celgene, which are all unrelated to this work. JLK and ZY declare no conflicts of interest.
Figures

References
-
- Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient‐reported symptoms from the Quality‐of‐Life Questionnaire C30. J Clin Oncol. 2004;22(17):3485‐3490. - PubMed
-
- Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire‐based study. Lancet Oncol. 2006;7(11):903‐909. - PubMed
-
- Di Maio M, Gallo C, Leighl NB, et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol. 2015;33(8):910‐915. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

