Health Profile of Preterm Males With Duchenne Muscular Dystrophy
- PMID: 34677095
- PMCID: PMC10928516
- DOI: 10.1177/08830738211047019
Health Profile of Preterm Males With Duchenne Muscular Dystrophy
Abstract
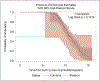
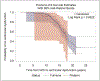
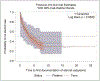
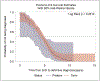
In this retrospective cohort study, we characterize the health profile of preterm males with Duchenne muscular dystrophy. Major clinical milestones (ambulation cessation, assisted ventilation use, and onset of left ventricular dysfunction) and corticosteroids use in males with Duchenne muscular dystrophy identified through a population-based surveillance system were analyzed using Kaplan-Meier survival curves and Cox proportional hazards modeling. The adjusted risk of receiving any respiratory intervention among preterm males with Duchenne muscular dystrophy was 87% higher than among the corresponding full-term males with Duchenne muscular dystrophy. The adjusted risks for ambulation cessation and left ventricular dysfunction were modestly elevated among preterm compared to full-term males, but the 95% confidence intervals contained the null. No difference in the start of corticosteroid use between preterm and full-term Duchenne muscular dystrophy males was observed. Overall, the disease course seems to be similar between preterm and full-term males with Duchenne muscular dystrophy; however, pulmonary function seems to be affected earlier among preterm males with Duchenne muscular dystrophy.
Keywords: Duchenne muscular dystrophy; children epidemiology; pediatric; preterm.
Conflict of interest statement
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9(1):77–93. - PubMed
-
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50(3):509–517. - PubMed
-
- Yiu EM, Kornberg AJ. Duchenne muscular dystrophy. Neurol India. 2008;56(3):236–247. - PubMed
-
- Zebracki K, Drotar D. Pain and activity limitations in children with Duchenne or Becker muscular dystrophy. Dev Med Child Neurol. 2008;50(7):546–552. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

