Intestinal goblet cells sample and deliver lumenal antigens by regulated endocytic uptake and transcytosis
- PMID: 34677124
- PMCID: PMC8594945
- DOI: 10.7554/eLife.67292
Intestinal goblet cells sample and deliver lumenal antigens by regulated endocytic uptake and transcytosis
Abstract
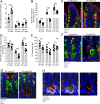
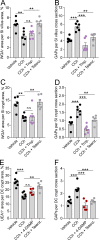
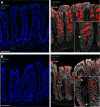
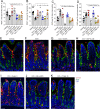
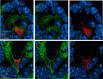
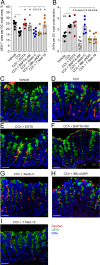
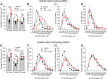
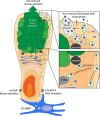
Intestinal goblet cells maintain the protective epithelial barrier through mucus secretion and yet sample lumenal substances for immune processing through formation of goblet cell associated antigen passages (GAPs). The cellular biology of GAPs and how these divergent processes are balanced and regulated by goblet cells remains unknown. Using high-resolution light and electron microscopy, we found that in mice, GAPs were formed by an acetylcholine (ACh)-dependent endocytic event remarkable for delivery of fluid-phase cargo retrograde into the trans-golgi network and across the cell by transcytosis - in addition to the expected transport of fluid-phase cargo by endosomes to multi-vesicular bodies and lysosomes. While ACh also induced goblet cells to secrete mucins, ACh-induced GAP formation and mucin secretion were functionally independent and mediated by different receptors and signaling pathways, enabling goblet cells to differentially regulate these processes to accommodate the dynamically changing demands of the mucosal environment for barrier maintenance and sampling of lumenal substances.
Keywords: antigen sampling; cell biology; goblet cells; immunology; inflammation; intestine; mouse.
Plain language summary
Cells in the gut need to be protected against the many harmful microbes which inhabit this environment. Yet the immune system also needs to ‘keep an eye’ on intestinal contents to maintain tolerance to innocuous substances, such as those from the diet. The ‘goblet cells’ that are part of the gut lining do both: they create a mucus barrier that stops germs from invading the body, but they also can pass on molecules from the intestine to immune cells deep in the tissue to promote tolerance. This is achieved through a ‘GAP’ mechanism. A chemical messenger called acetylcholine can trigger both mucus release and the GAP process in goblet cells. Gustafsson et al. investigated how the cells could take on these two seemingly opposing roles in response to the same signal. A fluorescent molecule was introduced into the intestines of mice, and monitored as it pass through the goblet cells. This revealed how the GAP process took place: the cells were able to capture molecules from the intestines, wrap them in internal sack-like vesicles and then transport them across the entire cell. To explore the role of acetylcholine, Gustafsson et al. blocked the receptors that detect the messenger at the surface of goblet cells. Different receptors and therefore different cascades of molecular events were found to control mucus secretion and GAP formation; this explains how the two processes can be performed in parallel and independently from each other. Understanding how cells relay molecules to the immune system is relevant to other tissues in contact with the environment, such as the eyes, the airways, or the inside of the genital and urinary tracts. Understanding, and then ultimately harnessing this mechanism could help design of new ways to deliver drugs to the immune system and alter immune outcomes.
© 2021, Gustafsson et al.
Conflict of interest statement
JG, JD, TR, KM, DK, KK, SH, JF, WL, RN No competing interests declared
Figures


Similar articles
-
Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon.Mucosal Immunol. 2015 Jan;8(1):198-210. doi: 10.1038/mi.2014.58. Epub 2014 Jul 9. Mucosal Immunol. 2015. PMID: 25005358 Free PMC article.
-
Autofluorescence imaging permits label-free cell type assignment and reveals the dynamic formation of airway secretory cell associated antigen passages (SAPs).Elife. 2023 Mar 30;12:e84375. doi: 10.7554/eLife.84375. Elife. 2023. PMID: 36994985 Free PMC article.
-
Goblet cells: multifaceted players in immunity at mucosal surfaces.Mucosal Immunol. 2018 Nov;11(6):1551-1557. doi: 10.1038/s41385-018-0039-y. Epub 2018 Jun 4. Mucosal Immunol. 2018. PMID: 29867079 Free PMC article. Review.
-
The relationship between intestinal goblet cells and the immune response.Biosci Rep. 2020 Oct 30;40(10):BSR20201471. doi: 10.1042/BSR20201471. Biosci Rep. 2020. PMID: 33017020 Free PMC article. Review.
-
An optimized method to visualize the goblet cell-associated antigen passages and identify goblet cells in the intestine, conjunctiva, and airway.Immunobiology. 2022 Nov;227(6):152260. doi: 10.1016/j.imbio.2022.152260. Epub 2022 Aug 17. Immunobiology. 2022. PMID: 36058107
Cited by
-
Intestinal Barrier Function and Neurodegenerative Disease.CNS Neurol Disord Drug Targets. 2024;23(9):1134-1142. doi: 10.2174/0118715273264097231116103948. CNS Neurol Disord Drug Targets. 2024. PMID: 38008941 Review.
-
The Diagnostic Potential of the Human Blood Microbiome: Are We Dreaming or Awake?Int J Mol Sci. 2023 Jun 21;24(13):10422. doi: 10.3390/ijms241310422. Int J Mol Sci. 2023. PMID: 37445600 Free PMC article. Review.
-
Bile Acids, Intestinal Barrier Dysfunction, and Related Diseases.Cells. 2023 Jul 19;12(14):1888. doi: 10.3390/cells12141888. Cells. 2023. PMID: 37508557 Free PMC article. Review.
-
Intestinal Mucosal Immune Barrier: A Powerful Firewall Against Severe Acute Pancreatitis-Associated Acute Lung Injury via the Gut-Lung Axis.J Inflamm Res. 2024 Apr 10;17:2173-2193. doi: 10.2147/JIR.S448819. eCollection 2024. J Inflamm Res. 2024. PMID: 38617383 Free PMC article. Review.
-
Role of Muscarinic Acetylcholine Receptors in Intestinal Epithelial Homeostasis: Insights for the Treatment of Inflammatory Bowel Disease.Int J Mol Sci. 2023 Mar 30;24(7):6508. doi: 10.3390/ijms24076508. Int J Mol Sci. 2023. PMID: 37047478 Free PMC article. Review.
References
-
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang K-T, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. - DOI - PMC - PubMed
-
- Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, Kannan MS. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of CD38/cyclic adenosine diphosphate ribose pathway. American Journal of Respiratory Cell and Molecular Biology. 2004;31:36–42. doi: 10.1165/rcmb.2003-0313OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

