Acute asthma exacerbation after SARS-CoV-2 vaccine (Sinovac®): a case report
- PMID: 34677155
- PMCID: PMC8562397
- DOI: 10.4103/2045-9912.326003
Acute asthma exacerbation after SARS-CoV-2 vaccine (Sinovac®): a case report
Abstract
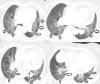
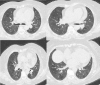
A 76-year-old female received a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine (CoronaVac, Sinovac®, Beijing, China) and subsequently experienced chest discomfort. A computed tomography performed 1 day after vaccination showed multiple infiltrations in both lungs and ground-glass shadows in both lung fields. Her fingertip oxygen saturation was 81% and there was widespread wheezing on physical examination. Based on these findings, the patient was hospitalized with a preliminary diagnosis of drug-induced pneumonitis and acute asthma exacerbation due to a SARS-CoV-2 vaccine. During her hospitalization, 40 mg/d systemic steroid, 4 times a day salbutamol nebulized, 2 L/min inhaled oxygen therapy and 400 mg/d moxifloxacin intravenous were administered for 5 days. One month later, the thorax computed tomography scan revealed that the previous findings were almost completely regressed.
Keywords: SARS-CoV-2; adverse event; allergy; computed tomography; infiltrations; side effect; systemic inflammation; vaccine.
Conflict of interest statement
No.
Figures

References
-
- Palacios R, Patiño EG, de Oliveira Piorelli R, et al. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac - PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:853. - PMC - PubMed
-
- World Health Organization. The Sinovac COVID-19 vaccine: What you need to know. [Accessed by July 27, 2021]. https://www.who.int/news-room/feature-stories/detail/the-sinovac-covid- ....
-
- Baraniuk C. What do we know about China’s covid-19 vaccines. BMJ? 2021;373:n912. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

