Optical Biosensor Platforms Display Varying Sensitivity for the Direct Detection of Influenza RNA
- PMID: 34677323
- PMCID: PMC8534094
- DOI: 10.3390/bios11100367
Optical Biosensor Platforms Display Varying Sensitivity for the Direct Detection of Influenza RNA
Abstract
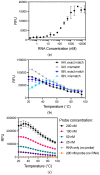
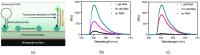
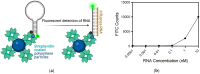
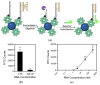
Detection methods that do not require nucleic acid amplification are advantageous for viral diagnostics due to their rapid results. These platforms could provide information for both accurate diagnoses and pandemic surveillance. Influenza virus is prone to pandemic-inducing genetic mutations, so there is a need to apply these detection platforms to influenza diagnostics. Here, we analyzed the Fast Evaluation of Viral Emerging Risks (FEVER) pipeline on ultrasensitive detection platforms, including a waveguide-based optical biosensor and a flow cytometry bead-based assay. The pipeline was also evaluated in silico for sequence coverage in comparison to the U.S. Centers for Disease Control and Prevention's (CDC) influenza A and B diagnostic assays. The influenza FEVER probe design had a higher tolerance for mismatched bases than the CDC's probes, and the FEVER probes altogether had a higher detection rate for influenza isolate sequences from GenBank. When formatted for use as molecular beacons, the FEVER probes detected influenza RNA as low as 50 nM on the waveguide-based optical biosensor and 1 nM on the flow cytometer. In addition to molecular beacons, which have an inherently high background signal we also developed an exonuclease selection method that could detect 500 pM of RNA. The combination of high-coverage probes developed using the FEVER pipeline coupled with ultrasensitive optical biosensors is a promising approach for future influenza diagnostic and biosurveillance applications.
Keywords: RNA; biosensor; detection; diagnostics; flow cytometer; influenza; waveguide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization Influenza (Seasonal) [(accessed on 8 February 2018)]. Available online: www.who.int/mediacentre/factsheets/fs211/en.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

