Applications of Microfluidics in Liquid Crystal-Based Biosensors
- PMID: 34677341
- PMCID: PMC8534167
- DOI: 10.3390/bios11100385
Applications of Microfluidics in Liquid Crystal-Based Biosensors
Abstract
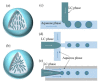
Liquid crystals (LCs) with stimuli-responsive configuration transition and optical anisotropic properties have attracted enormous interest in the development of simple and label-free biosensors. The combination of microfluidics and the LCs offers great advantages over traditional LC-based biosensors including small sample consumption, fast analysis and low cost. Moreover, microfluidic techniques provide a promising tool to fabricate uniform and reproducible LC-based sensing platforms. In this review, we emphasize the recent development of microfluidics in the fabrication and integration of LC-based biosensors, including LC planar sensing platforms and LC droplets. Fabrication and integration of LC-based planar platforms with microfluidics for biosensing applications are first introduced. The generation and entrapment of monodisperse LC droplets with different microfluidic structures, as well as their applications in the detection of chemical and biological species, are then summarized. Finally, the challenges and future perspectives of the development of LC-based microfluidic biosensors are proposed. This review will promote the understanding of microfluidic techniques in LC-based biosensors and facilitate the development of LC-based microfluidic biosensing devices with high performance.
Keywords: biosensors; liquid crystal droplets; liquid crystal planar interface; liquid crystals; microfluidics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- He Z., Gou F., Chen R., Yin K., Zhan T., Wu S.T. Liquid crystal beam steering devices: Principles, recent advances, and future developments. Crystals. 2019;9:292. doi: 10.3390/cryst9060292. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

