Liquid Biopsy-Based Biosensors for MRD Detection and Treatment Monitoring in Non-Small Cell Lung Cancer (NSCLC)
- PMID: 34677350
- PMCID: PMC8533977
- DOI: 10.3390/bios11100394
Liquid Biopsy-Based Biosensors for MRD Detection and Treatment Monitoring in Non-Small Cell Lung Cancer (NSCLC)
Abstract
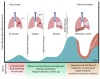
Globally, non-small cell lung cancer (NSCLC) is the leading cause of cancer deaths. Despite advancements in chemotherapy and targeted therapies, the 5-year survival rate has remained at 16% for the past forty years. Minimal residual disease (MRD) is described as the existence of either isolated tumour cells or circulating tumour cells in biological liquid of patients after removal of the primary tumour without any clinical signs of cancer. Recently, liquid biopsy has been promising as a non-invasive method of disease monitoring and treatment guidelines as an MRD marker. Liquid biopsy could be used to detect and assess earlier stages of NSCLC, post-treatment MRD, resistance to targeted therapies, immune checkpoint inhibitors (ICIs) and tumour mutational burden. MRD surveillance has been proposed as a potential marker for lung cancer relapse. Principally, biosensors provide the quantitative analysis of various materials by converting biological functions into quantifiable signals. Biosensors are usually operated to detect antibodies, enzymes, DNA, RNA, extracellular vesicles (EVs) and whole cells. Here, we present a category of biosensors based on the signal transduction method for identifying biosensor-based biomarkers in liquid biopsy specimens to monitor lung cancer treatment.
Keywords: bio-sensor; liquid biopsy; minimal residual disease; non-small cell lung cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Goldstraw P., Crowley J., Chansky K., Giroux D.J., Groome P.A., Rami-Porta R., Postmus P., Rusch V., Sobin L. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J. Thorac. Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

