Recent Developments in Nanoporous Graphene Membranes for Organic Solvent Nanofiltration: A Short Review
- PMID: 34677558
- PMCID: PMC8538602
- DOI: 10.3390/membranes11100793
Recent Developments in Nanoporous Graphene Membranes for Organic Solvent Nanofiltration: A Short Review
Abstract
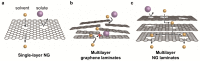
Graphene-based membranes are promising candidates for efficient organic solvent nanofiltration (OSN) processes because of their unique structural characteristics, such as mechanical/chemical stability and precise molecular sieving. Recently, to improve organic solvent permeance and selectivity, nanopores have been fabricated on graphene planes via chemical and physical methods. The nanopores serve as an additional channel for facilitating ultrafast solvent permeation while filtering organic molecules by size exclusion. This review summarizes the recent developments in nanoporous graphene (NG)-based membranes for OSN applications. The membranes are categorized depending on the membrane structure: single-layer NG, multilayer NG, and graphene-based composite membranes hybridized with other porous materials. Techniques for nanopore generation on graphene, as well as the challenges faced and the perspectives required for the commercialization of NG membranes, are also discussed.
Keywords: graphene; membrane; nanopore; organic solvent nanofiltration; separation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Nie L.A., Chuah C.Y., Bae T.H., Lee J.M. Graphene-Based Advanced Membrane Applications in Organic Solvent Nanofiltration. Adv. Funct. Mater. 2021;31:2006949. doi: 10.1002/adfm.202006949. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

