Spontaneous Cleavage at Glu and Gln Residues in Long-Lived Proteins
- PMID: 34677941
- PMCID: PMC9206231
- DOI: 10.1021/acschembio.1c00379
Spontaneous Cleavage at Glu and Gln Residues in Long-Lived Proteins
Abstract
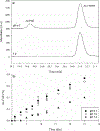
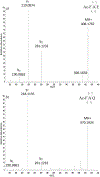
Long-lived proteins (LLPs) are prone to deterioration with time, and one prominent breakdown process is the scission of peptide bonds. These cleavages can either be enzymatic or spontaneous. In this study, human lens proteins were examined and many were found to have been cleaved on the C-terminal side of Glu and Gln residues. Such cleavages could be reproduced experimentally by in vitro incubation of Glu- or Gln-containing peptides at physiological pHs. Spontaneous cleavage was dependent on pH and amino acid sequence. These model peptide studies suggested that the mechanism involves a cyclic intermediate and is therefore analogous to that characterized for cleavage of peptide bonds adjacent to Asp and Asn residues. An increased amount of some Glu/Gln cleaved peptides in the insoluble fraction of human lenses suggests that cleavage may act to destabilize proteins. Spontaneous cleavage at Glu and Gln, as well as recently described cross-linking at these residues, can therefore be added to the similar processes affecting long-lived proteins that have already been documented for Asn and Asp residues.
Conflict of interest statement
Figures

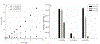
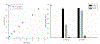
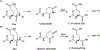
References
-
- Truscott RJW, Long-Lived Cells and Long-Lived Proteins in the Human Body, in Long-lived Proteins in Human Aging and Disease. 2021. p. 5–42.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

