NLRP3 inflammasome activation triggers gasdermin D-independent inflammation
- PMID: 34678046
- PMCID: PMC8780201
- DOI: 10.1126/sciimmunol.abj3859
NLRP3 inflammasome activation triggers gasdermin D-independent inflammation
Abstract
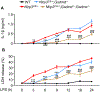
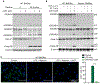
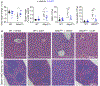
NOD-like receptor (NLR), family pyrin domain containing 3 (NLRP3) assembles a protein complex known as the NLRP3 inflammasome upon sensing certain pathogen products or sterile danger signals. Gain-of-function mutations such as the D301N substitution in NLRP3, which cause its constitutive activation (NLRP3CA) also results in inflammasome assembly. This inflammasome processes pro–interleukin-1 β (pro–IL-1β) and pro–IL-18 into bioactive IL-1β and IL-18, respectively, and cleaves gasdermin D (GSDMD). GSDMD amino-terminal fragments form plasma membrane pores that facilitate the secretion of IL-1β and IL-18 and lead to the inflammatory cell death pyroptosis. Accordingly, GSDMD inactivation results in negligible spontaneous inflammation in various experimental models such as in Nlrp3CA/+ mice lacking GSDMD (Nlrp3CA/+;Gsdmd−/− mice). Here, we found that Nlrp3CA/+;Gsdmd−/− mice, when challenged with LPS or TNF-α, still secreted IL-1β and IL-18, indicating inflammasome activation independent of GSDMD. Accordingly, Gsdmd−/− macrophages failed to secrete IL-1β and undergo pyroptosis when briefly exposed to NLRP3 inflammasome activators but released these cytokines when persistently activated. Sustained NLRP3 inflammasome induced caspase-8/-3 and GSDME cleavage and IL-1β maturation in vitro in Gsdmd−/− macrophages. Thus, a salvage inflammatory pathway involving caspase-8/-3–GSDME was activated after NLRP3 activation when the canonical NLRP3-GSDMD signaling was blocked. Consistent with genetic data, the active metabolite of FDA-approved disulfiram CuET, which inhibited GSDMD and GSDME cleavage in macrophages, reduced the severe inflammation and tissue damage that occurred in the Nlrp3CA/+ mice. Thus, NLRP3 inflammasome activation overwhelms the protection afforded by GSDMD deficiency, rewiring signaling cascades through mechanisms that include GSDME to propagate inflammation.
Figures





References
-
- Schroder K, Tschopp J, The inflammasomes. Cell 140, 821–832 (2010). - PubMed
-
- Broz P, Dixit VM, Inflammasomes: mechanism of assembly, regulation and signalling. Nature reviews. Immunology 16, 407–420 (2016). - PubMed
-
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F, Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). - PubMed
-
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang D-C, Shao F, Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

