Serial assessment of measurable residual disease in medulloblastoma liquid biopsies
- PMID: 34678152
- PMCID: PMC9620970
- DOI: 10.1016/j.ccell.2021.09.012
Serial assessment of measurable residual disease in medulloblastoma liquid biopsies
Abstract
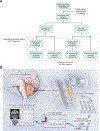
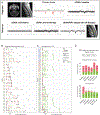
Nearly one-third of children with medulloblastoma, a malignant embryonal tumor of the cerebellum, succumb to their disease. Conventional response monitoring by imaging and cerebrospinal fluid (CSF) cytology remains challenging, and a marker for measurable residual disease (MRD) is lacking. Here, we show the clinical utility of CSF-derived cell-free DNA (cfDNA) as a biomarker of MRD in serial samples collected from children with medulloblastoma (123 patients, 476 samples) enrolled on a prospective trial. Using low-coverage whole-genome sequencing, tumor-associated copy-number variations in CSF-derived cfDNA are investigated as an MRD surrogate. MRD is detected at baseline in 85% and 54% of patients with metastatic and localized disease, respectively. The number of MRD-positive patients declines with therapy, yet those with persistent MRD have significantly higher risk of progression. Importantly, MRD detection precedes radiographic progression in half who relapse. Our findings advocate for the prospective assessment of CSF-derived liquid biopsies in future trials for medulloblastoma.
Keywords: biomarkers; cell-free DNA; cerebrospinal fluid; childhood cancer; liquid biopsy; measurable residual disease; medulloblastoma; microscopic residual disease; minimal residual disease; relapse disease.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Comment in
-
SEQing to find hidden medulloblastoma cells.Cancer Cell. 2021 Nov 8;39(11):1452-1454. doi: 10.1016/j.ccell.2021.09.014. Epub 2021 Oct 21. Cancer Cell. 2021. PMID: 34678149
-
Cerebrospinal fluid liquid biopsies for medulloblastoma.Nat Rev Clin Oncol. 2022 Feb;19(2):73-74. doi: 10.1038/s41571-021-00590-1. Nat Rev Clin Oncol. 2022. PMID: 34912051 No abstract available.
References
-
- Chang CH, Housepian EM, and Herbert C (1969). An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology 93, 1351–1359. - PubMed
-
- Coustan-Smith E, Sancho J, Hancock ML, Boyett JM, Behm FG, Raimondi SC, Sandlund JT, Rivera GK, Rubnitz JE, and Ribeiro RC (2000). Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood 96, 2691–2696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

