A prospective prostate cancer screening programme for men with pathogenic variants in mismatch repair genes (IMPACT): initial results from an international prospective study
- PMID: 34678156
- PMCID: PMC8576477
- DOI: 10.1016/S1470-2045(21)00522-2
A prospective prostate cancer screening programme for men with pathogenic variants in mismatch repair genes (IMPACT): initial results from an international prospective study
Abstract
Background: Lynch syndrome is a rare familial cancer syndrome caused by pathogenic variants in the mismatch repair genes MLH1, MSH2, MSH6, or PMS2, that cause predisposition to various cancers, predominantly colorectal and endometrial cancer. Data are emerging that pathogenic variants in mismatch repair genes increase the risk of early-onset aggressive prostate cancer. The IMPACT study is prospectively assessing prostate-specific antigen (PSA) screening in men with germline mismatch repair pathogenic variants. Here, we report the usefulness of PSA screening, prostate cancer incidence, and tumour characteristics after the first screening round in men with and without these germline pathogenic variants.
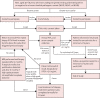
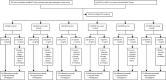
Methods: The IMPACT study is an international, prospective study. Men aged 40-69 years without a previous prostate cancer diagnosis and with a known germline pathogenic variant in the MLH1, MSH2, or MSH6 gene, and age-matched male controls who tested negative for a familial pathogenic variant in these genes were recruited from 34 genetic and urology clinics in eight countries, and underwent a baseline PSA screening. Men who had a PSA level higher than 3·0 ng/mL were offered a transrectal, ultrasound-guided, prostate biopsy and a histopathological analysis was done. All participants are undergoing a minimum of 5 years' annual screening. The primary endpoint was to determine the incidence, stage, and pathology of screening-detected prostate cancer in carriers of pathogenic variants compared with non-carrier controls. We used Fisher's exact test to compare the number of cases, cancer incidence, and positive predictive values of the PSA cutoff and biopsy between carriers and non-carriers and the differences between disease types (ie, cancer vs no cancer, clinically significant cancer vs no cancer). We assessed screening outcomes and tumour characteristics by pathogenic variant status. Here we present results from the first round of PSA screening in the IMPACT study. This study is registered with ClinicalTrials.gov, NCT00261456, and is now closed to accrual.
Findings: Between Sept 28, 2012, and March 1, 2020, 828 men were recruited (644 carriers of mismatch repair pathogenic variants [204 carriers of MLH1, 305 carriers of MSH2, and 135 carriers of MSH6] and 184 non-carrier controls [65 non-carriers of MLH1, 76 non-carriers of MSH2, and 43 non-carriers of MSH6]), and in order to boost the sample size for the non-carrier control groups, we randomly selected 134 non-carriers from the BRCA1 and BRCA2 cohort of the IMPACT study, who were included in all three non-carrier cohorts. Men were predominantly of European ancestry (899 [93%] of 953 with available data), with a mean age of 52·8 years (SD 8·3). Within the first screening round, 56 (6%) men had a PSA concentration of more than 3·0 ng/mL and 35 (4%) biopsies were done. The overall incidence of prostate cancer was 1·9% (18 of 962; 95% CI 1·1-2·9). The incidence among MSH2 carriers was 4·3% (13 of 305; 95% CI 2·3-7·2), MSH2 non-carrier controls was 0·5% (one of 210; 0·0-2·6), MSH6 carriers was 3·0% (four of 135; 0·8-7·4), and none were detected among the MLH1 carriers, MLH1 non-carrier controls, and MSH6 non-carrier controls. Prostate cancer incidence, using a PSA threshold of higher than 3·0 ng/mL, was higher in MSH2 carriers than in MSH2 non-carrier controls (4·3% vs 0·5%; p=0·011) and MSH6 carriers than MSH6 non-carrier controls (3·0% vs 0%; p=0·034). The overall positive predictive value of biopsy using a PSA threshold of 3·0 ng/mL was 51·4% (95% CI 34·0-68·6), and the overall positive predictive value of a PSA threshold of 3·0 ng/mL was 32·1% (20·3-46·0).
Interpretation: After the first screening round, carriers of MSH2 and MSH6 pathogenic variants had a higher incidence of prostate cancer compared with age-matched non-carrier controls. These findings support the use of targeted PSA screening in these men to identify those with clinically significant prostate cancer. Further annual screening rounds will need to confirm these findings.
Funding: Cancer Research UK, The Ronald and Rita McAulay Foundation, the National Institute for Health Research support to Biomedical Research Centres (The Institute of Cancer Research and Royal Marsden NHS Foundation Trust; Oxford; Manchester and the Cambridge Clinical Research Centre), Mr and Mrs Jack Baker, the Cancer Council of Tasmania, Cancer Australia, Prostate Cancer Foundation of Australia, Cancer Council of Victoria, Cancer Council of South Australia, the Victorian Cancer Agency, Cancer Australia, Prostate Cancer Foundation of Australia, Asociación Española Contra el Cáncer (AECC), the Instituto de Salud Carlos III, Fondo Europeo de Desarrollo Regional (FEDER), the Institut Català de la Salut, Autonomous Government of Catalonia, Fundação para a Ciência e a Tecnologia, National Institutes of Health National Cancer Institute, Swedish Cancer Society, General Hospital in Malmö Foundation for Combating Cancer.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests HL holds patents on intact PSA assays and is named on a patent for a statistical method to detect prostate cancer licensed to Arctic Partners and commercialised by OPKO Health, and has stock in Arctic Partners and OPKO Health and receives royalties from sales of the 4Kscore test. RAE has received speaker honoraria from Genitourinary-American Society of Clinical Oncology, The University of Chicago, European Society for Medical Oncology (paid by Bayer and Ipsen), and The Royal Marsden NHS Foundation Trust (with support from Janssen), and is a member of the AstraZeneca UK Limited Prostate Dx Advisory Panel external expert committee. No organisation had any role in the decision to publish or in the writing of the manuscript. All other authors declare no competing interests.
Figures
Comment in
-
Reimagining prostate cancer screening: the IMPACT of germline mutations.Lancet Oncol. 2021 Nov;22(11):1491-1492. doi: 10.1016/S1470-2045(21)00571-4. Epub 2021 Oct 19. Lancet Oncol. 2021. PMID: 34678157 No abstract available.
-
Re: A Prospective Prostate Cancer Screening Programme for Men with Pathogenic Variants in Mismatch Repair Genes (IMPACT): Initial Results from an International Prospective Study.Eur Urol. 2022 Feb;81(2):216-218. doi: 10.1016/j.eururo.2021.11.030. Epub 2021 Dec 9. Eur Urol. 2022. PMID: 34895922 No abstract available.
-
Urological Oncology: Prostate Cancer.J Urol. 2022 Jun;207(6):1348-1351. doi: 10.1097/JU.0000000000002511. Epub 2022 Mar 17. J Urol. 2022. PMID: 35296140 No abstract available.
References
-
- Barrow PJ, Ingham S, O’Hara C. The spectrum of urological malignancy in Lynch syndrome. Fam Cancer. 2013;12:57–63. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

