Efficacy of typhoid conjugate vaccine in Nepal: final results of a phase 3, randomised, controlled trial
- PMID: 34678198
- PMCID: PMC8551681
- DOI: 10.1016/S2214-109X(21)00346-6
Efficacy of typhoid conjugate vaccine in Nepal: final results of a phase 3, randomised, controlled trial
Abstract
Background: Typhoid fever is a major public health problem in low-resource settings. Vaccination can help curb the disease and might reduce transmission. We have previously reported an interim analysis of the efficacy of typhoid conjugate vaccine (TCV) in Nepali children. Here we report the final results after 2 years of follow-up.
Methods: We did a participant-masked and observer-masked individually randomised trial in Lalitpur, Nepal, in which 20 019 children aged 9 months to younger than 16 years were randomly assigned in a 1:1 ratio to receive a single dose of TCV (Typbar TCV, Bharat Biotech International, India) or capsular group A meningococcal conjugate vaccine (MenA). Participants were followed up until April 9, 2020. The primary outcome was blood culture-confirmed typhoid fever. Cases were captured via passive surveillance and active telephone surveillance followed by medical record review. The trial is registered at ISRCTN registry, ISRCTN43385161 and is ongoing.
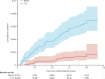
Findings: From Nov 20, 2017, to April 9, 2018, of 20 119 children screened, 20 019 participants were randomly assigned to receive TCV or MenA vaccine. There were 75 cases of blood culture-confirmed typhoid fever included in the analysis (13 in the TCV group and 62 in the MenA group) over the 2-year period. The protective efficacy of TCV against blood culture-confirmed typhoid fever at 2 years was 79·0% (95% CI 61·9-88·5; p<0·0001). The incidence of typhoid fever was 72 (95% CI 38-123) cases per 100 000 person-years in the TCV group and 342 (95% CI 262-438) cases per 100 000 person-years in the MenA group. Adverse events occurring within the first 7 days post-vaccination were reported previously.
Interpretation: The final results of this randomised, controlled trial are in keeping with the results of our published interim analysis. There is no evidence of waning protection over a 2-year period. These findings add further support for the WHO recommendations on control of enteric fever.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AJP is Chair of the UK Department of Health and Social Care's (DHSC) Joint Committee on Vaccination & Immunisation (JCVI) and is a member of WHO's Strategic Advisory Group of Experts on Immunization. AJP is a National Institute for Health Research (NIHR) Senior Investigator. The views expressed in this article do not necessarily represent the views of DHSC, JCVI, NIHR, or WHO. AJP is chief investigator on clinical trials of Oxford University's COVID-19 vaccine funded by NIHR. Oxford University is in a joint COVID-19 vaccine development partnership with AstraZeneca. VEP is a member of the WHO Immunization and Vaccine-related Implementation Research Advisory Committee and has received reimbursement from Merck and Pfizer for travel expenses to attend scientific input engagements unrelated to typhoid vaccines. All other authors declare that they have no conflict of interest.
Figures
References
-
- Mogasale V, Maskery B, Ochiai RL. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2:e570–e580. - PubMed
-
- Global Burden of Disease Collaborative Network . GBD 2020 Cause and Risk Summaries: Typhoid fever — Level 4 cause. Institute for Health Metrics and Evaluation (IHME); Seattle, WA: 2020. http://www.healthdata.org/results/gbd_summaries/2019/typhoid-fever-level...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous