Pluripotent stem cell derived dopaminergic subpopulations model the selective neuron degeneration in Parkinson's disease
- PMID: 34678205
- PMCID: PMC8581055
- DOI: 10.1016/j.stemcr.2021.09.014
Pluripotent stem cell derived dopaminergic subpopulations model the selective neuron degeneration in Parkinson's disease
Abstract
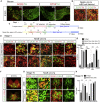
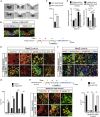
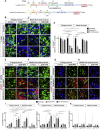
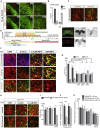
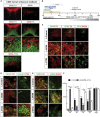
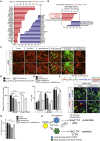
In Parkinson's disease (PD), substantia nigra (SN) dopaminergic (DA) neurons degenerate, while related ventral tegmental area (VTA) DA neurons remain relatively unaffected. Here, we present a methodology that directs the differentiation of mouse and human pluripotent stem cells toward either SN- or VTA-like DA lineage and models their distinct vulnerabilities. We show that the level of WNT activity is critical for the induction of the SN- and VTA-lineage transcription factors Sox6 and Otx2, respectively. Both WNT signaling modulation and forced expression of these transcription factors can drive DA neurons toward the SN- or VTA-like fate. Importantly, the SN-like lineage enriched DA cultures recapitulate the selective sensitivity to mitochondrial toxins as observed in PD, while VTA-like neuron-enriched cultures are more resistant. Furthermore, a proteomics approach led to the identification of compounds that alter SN neuronal survival, demonstrating the utility of our strategy for disease modeling and drug discovery.
Keywords: derivation of substantia nigra dopaminergic neuronal lineage from pluripotent stem cells; directed differentiation of pluripotent stem cells into distinct dopaminergic subpopulations; modeling selective dopaminergic vulnerability in vitro; pluripotent stem cell-based model of Parkinson's disease.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Blaess S., Bodea G.O., Kabanova A., Chanet S., Mugniery E., Derouiche A., Stephen D., Joyner A.L. Temporal-spatial changes in Sonic Hedgehog expression and signaling reveal different potentials of ventral mesencephalic progenitors to populate distinct ventral midbrain nuclei. Neural Dev. 2011;6:29. - PMC - PubMed
-
- Blum M. A null mutation in TGF-alpha leads to a reduction in midbrain dopaminergic neurons in the substantia nigra. Nat. Neurosci. 1998;1:374–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

