Structural determinants of the integrin transmembrane domain required for bidirectional signal transmission across the cell membrane
- PMID: 34678312
- PMCID: PMC8569584
- DOI: 10.1016/j.jbc.2021.101318
Structural determinants of the integrin transmembrane domain required for bidirectional signal transmission across the cell membrane
Abstract
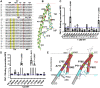
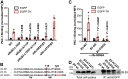
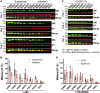
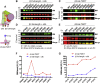
Studying the tight activity regulation of platelet-specific integrin αIIbβ3 is foundational and paramount to our understanding of integrin structure and activation. αIIbβ3 is essential for the aggregation and adhesion function of platelets in hemostasis and thrombosis. Structural and mutagenesis studies have previously revealed the critical role of αIIbβ3 transmembrane (TM) association in maintaining the inactive state. Gain-of-function TM mutations were identified and shown to destabilize the TM association leading to integrin activation. Studies using isolated TM peptides have suggested an altered membrane embedding of the β3 TM α-helix coupled with αIIbβ3 activation. However, controversies remain as to whether and how the TM α-helices change their topologies in the context of full-length integrin in native cell membrane. In this study, we utilized proline scanning mutagenesis and cysteine scanning accessibility assays to analyze the structure and function correlation of the αIIbβ3 TM domain. Our identification of loss-of-function proline mutations in the TM domain suggests the requirement of a continuous TM α-helical structure in transmitting activation signals bidirectionally across the cell membrane, characterized by the inside-out activation for ligand binding and the outside-in signaling for cell spreading. Similar results were found for αLβ2 and α5β1 TM domains, suggesting a generalizable mechanism. We also detected a topology change of β3 TM α-helix within the cell membrane, but only under conditions of cell adhesion and the absence of αIIb association. Our data demonstrate the importance of studying the structure and function of the integrin TM domain in the native cell membrane.
Keywords: cell adhesion; integrin; platelet; talin; transmembrane domain.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest Authors declare that they have no competing interests.
Figures





Similar articles
-
Activation of platelet alphaIIbbeta3 by an exogenous peptide corresponding to the transmembrane domain of alphaIIb.J Biol Chem. 2006 Dec 1;281(48):36732-41. doi: 10.1074/jbc.M605877200. Epub 2006 Oct 10. J Biol Chem. 2006. PMID: 17032655
-
Talin-driven inside-out activation mechanism of platelet αIIbβ3 integrin probed by multimicrosecond, all-atom molecular dynamics simulations.Proteins. 2014 Dec;82(12):3231-3240. doi: 10.1002/prot.24540. Epub 2014 Sep 25. Proteins. 2014. PMID: 24677266 Free PMC article.
-
The structure of the integrin alphaIIbbeta3 transmembrane complex explains integrin transmembrane signalling.EMBO J. 2009 May 6;28(9):1351-61. doi: 10.1038/emboj.2009.63. Epub 2009 Mar 12. EMBO J. 2009. PMID: 19279667 Free PMC article.
-
Platelet integrin αIIbβ3: signal transduction, regulation, and its therapeutic targeting.J Hematol Oncol. 2019 Mar 7;12(1):26. doi: 10.1186/s13045-019-0709-6. J Hematol Oncol. 2019. PMID: 30845955 Free PMC article. Review.
-
Beta3 tyrosine phosphorylation in alphaIIbbeta3 (platelet membrane GP IIb-IIIa) outside-in integrin signaling.Thromb Haemost. 2001 Jul;86(1):246-58. Thromb Haemost. 2001. PMID: 11487013 Review.
Cited by
-
The mechanism by which enoxaparin sodium-high-viscosity bone cement reduces thrombosis by regulating CD40 expression in endothelial cells.BMC Musculoskelet Disord. 2022 May 30;23(1):513. doi: 10.1186/s12891-022-05469-5. BMC Musculoskelet Disord. 2022. PMID: 35637498 Free PMC article.
-
Intrinsic self-organization of integrin nanoclusters within focal adhesions is required for cellular mechanotransduction.bioRxiv [Preprint]. 2023 Nov 20:2023.11.20.567975. doi: 10.1101/2023.11.20.567975. bioRxiv. 2023. PMID: 38045378 Free PMC article. Preprint.
-
Molecular Modeling Insights into the Structure and Behavior of Integrins: A Review.Cells. 2023 Jan 14;12(2):324. doi: 10.3390/cells12020324. Cells. 2023. PMID: 36672259 Free PMC article. Review.
-
LncRNA MSTRG.22719.16 mediates the reduction of enoxaparin sodium high-viscosity bone cement-induced thrombosis by targeting the ocu-miR-326-5p/CD40 axis.J Orthop Surg Res. 2023 Sep 22;18(1):716. doi: 10.1186/s13018-023-04109-5. J Orthop Surg Res. 2023. PMID: 37736740 Free PMC article.
-
Analysis of Integrin αIIb Subunit Dynamics Reveals Long-Range Effects of Missense Mutations on Calf Domains.Int J Mol Sci. 2022 Jan 13;23(2):858. doi: 10.3390/ijms23020858. Int J Mol Sci. 2022. PMID: 35055046 Free PMC article.
References
-
- Hynes R.O. Integrins: Bi-directional, allosteric, signalling machines. Cell. 2002;110:673–687. - PubMed
-
- Kechagia J.Z., Ivaska J., Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019;20:457–473. - PubMed
-
- Kim C., Ye F., Ginsberg M.H. Regulation of integrin activation. Annu. Rev. Cell Dev. Biol. 2011;27:321–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

