Structural determinants of the integrin transmembrane domain required for bidirectional signal transmission across the cell membrane
- PMID: 34678312
- PMCID: PMC8569584
- DOI: 10.1016/j.jbc.2021.101318
Structural determinants of the integrin transmembrane domain required for bidirectional signal transmission across the cell membrane
Abstract
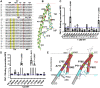
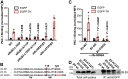
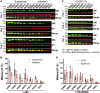
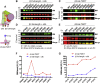
Studying the tight activity regulation of platelet-specific integrin αIIbβ3 is foundational and paramount to our understanding of integrin structure and activation. αIIbβ3 is essential for the aggregation and adhesion function of platelets in hemostasis and thrombosis. Structural and mutagenesis studies have previously revealed the critical role of αIIbβ3 transmembrane (TM) association in maintaining the inactive state. Gain-of-function TM mutations were identified and shown to destabilize the TM association leading to integrin activation. Studies using isolated TM peptides have suggested an altered membrane embedding of the β3 TM α-helix coupled with αIIbβ3 activation. However, controversies remain as to whether and how the TM α-helices change their topologies in the context of full-length integrin in native cell membrane. In this study, we utilized proline scanning mutagenesis and cysteine scanning accessibility assays to analyze the structure and function correlation of the αIIbβ3 TM domain. Our identification of loss-of-function proline mutations in the TM domain suggests the requirement of a continuous TM α-helical structure in transmitting activation signals bidirectionally across the cell membrane, characterized by the inside-out activation for ligand binding and the outside-in signaling for cell spreading. Similar results were found for αLβ2 and α5β1 TM domains, suggesting a generalizable mechanism. We also detected a topology change of β3 TM α-helix within the cell membrane, but only under conditions of cell adhesion and the absence of αIIb association. Our data demonstrate the importance of studying the structure and function of the integrin TM domain in the native cell membrane.
Keywords: cell adhesion; integrin; platelet; talin; transmembrane domain.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest Authors declare that they have no competing interests.
Figures





References
-
- Hynes R.O. Integrins: Bi-directional, allosteric, signalling machines. Cell. 2002;110:673–687. - PubMed
-
- Kechagia J.Z., Ivaska J., Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019;20:457–473. - PubMed
-
- Kim C., Ye F., Ginsberg M.H. Regulation of integrin activation. Annu. Rev. Cell Dev. Biol. 2011;27:321–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

