Progesterone receptor membrane component 1 (PGRMC1) binds and stabilizes cytochromes P450 through a heme-independent mechanism
- PMID: 34678314
- PMCID: PMC8591507
- DOI: 10.1016/j.jbc.2021.101316
Progesterone receptor membrane component 1 (PGRMC1) binds and stabilizes cytochromes P450 through a heme-independent mechanism
Abstract
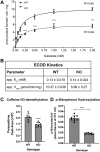
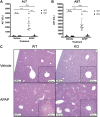
Progesterone receptor membrane component 1 (PGRMC1) is a heme-binding protein implicated in a wide range of cellular functions. We previously showed that PGRMC1 binds to cytochromes P450 in yeast and mammalian cells and supports their activity. Recently, the paralog PGRMC2 was shown to function as a heme chaperone. The extent of PGRMC1 function in cytochrome P450 biology and whether PGRMC1 is also a heme chaperone are unknown. Here, we examined the function of Pgrmc1 in mouse liver using a knockout model and found that Pgrmc1 binds and stabilizes a broad range of cytochromes P450 in a heme-independent manner. Proteomic and transcriptomic studies demonstrated that Pgrmc1 binds more than 13 cytochromes P450 and supports maintenance of cytochrome P450 protein levels posttranscriptionally. In vitro assays confirmed that Pgrmc1 KO livers exhibit reduced cytochrome P450 activity consistent with reduced enzyme levels. Mechanistic studies in cultured cells demonstrated that PGRMC1 stabilizes cytochromes P450 and that binding and stabilization do not require PGRMC1 binding to heme. Importantly, Pgrmc1-dependent stabilization of cytochromes P450 is physiologically relevant, as Pgrmc1 deletion protected mice from acetaminophen-induced liver injury. Finally, evaluation of Y113F mutant Pgrmc1, which lacks the axial heme iron-coordinating hydroxyl group, revealed that proper iron coordination is not required for heme binding, but is required for binding to ferrochelatase, the final enzyme in heme biosynthesis. PGRMC1 was recently identified as the causative mutation in X-linked isolated pediatric cataract formation. Together, these results demonstrate a heme-independent function for PGRMC1 in cytochrome P450 stability that may underlie clinical phenotypes.
Keywords: cytochrome P450; drug metabolism; enzyme degradation; heme; liver metabolism; protein turnover.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
S2R(Pgrmc1): the cytochrome-related sigma-2 receptor that regulates lipid and drug metabolism and hormone signaling.Expert Opin Drug Metab Toxicol. 2012 Mar;8(3):361-70. doi: 10.1517/17425255.2012.658367. Epub 2012 Feb 1. Expert Opin Drug Metab Toxicol. 2012. PMID: 22292588 Review.
-
PGRMC1: An enigmatic heme-binding protein.Pharmacol Ther. 2023 Jan;241:108326. doi: 10.1016/j.pharmthera.2022.108326. Epub 2022 Dec 1. Pharmacol Ther. 2023. PMID: 36463977 Free PMC article. Review.
-
Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes.Cell Metab. 2007 Feb;5(2):143-9. doi: 10.1016/j.cmet.2006.12.009. Cell Metab. 2007. PMID: 17276356
-
Cystathionine β-synthase and PGRMC1 as CO sensors.Free Radic Biol Med. 2016 Oct;99:333-344. doi: 10.1016/j.freeradbiomed.2016.08.025. Epub 2016 Aug 24. Free Radic Biol Med. 2016. PMID: 27565814 Review.
-
Spectroscopic and mutagenesis studies of human PGRMC1.Biochemistry. 2015 Mar 3;54(8):1638-47. doi: 10.1021/bi501177e. Epub 2015 Feb 23. Biochemistry. 2015. PMID: 25675345 Free PMC article.
Cited by
-
Mutant Samd9l expression impairs hematopoiesis and induces bone marrow failure in mice.J Clin Invest. 2022 Nov 1;132(21):e158869. doi: 10.1172/JCI158869. J Clin Invest. 2022. PMID: 36074606 Free PMC article.
-
The PGRMC1 Antagonist AG-205 Inhibits Synthesis of Galactosylceramide and Sulfatide.Cells. 2021 Dec 13;10(12):3520. doi: 10.3390/cells10123520. Cells. 2021. PMID: 34944026 Free PMC article.
-
The Mediator complex subunit MoMed15 plays an important role in conferring sensitivity to isoprothiolane by modulating xenobiotic metabolism in M. oryzae.mBio. 2024 Dec 11;15(12):e0177824. doi: 10.1128/mbio.01778-24. Epub 2024 Nov 12. mBio. 2024. PMID: 39530687 Free PMC article.
-
Protein subinteractomes of human microsomal cytochromes P450.Mol Biol Rep. 2025 Feb 12;52(1):226. doi: 10.1007/s11033-025-10341-5. Mol Biol Rep. 2025. PMID: 39937310 Review.
-
Fission yeast Dap1 heme iron-coordinating residue Y83 is required for cytochromes P450 function.MicroPubl Biol. 2022 Aug 23;2022:10.17912/micropub.biology.000631. doi: 10.17912/micropub.biology.000631. eCollection 2022. MicroPubl Biol. 2022. PMID: 36090151 Free PMC article.
References
-
- Ortiz de Montellano P.R., editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd Ed. Kluwer Academic/Plenum Publishers; New York, NY: 2005.
-
- Hughes A.L., Powell D.W., Bard M., Eckstein J., Barbuch R., Link A.J., Espenshade P.J. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007;5:143–149. - PubMed
-
- Peluso J.J., Liu X., Saunders M.M., Claffey K.P., Phoenix K. Regulation of ovarian cancer cell viability and sensitivity to cisplatin by progesterone receptor membrane component-1. J. Clin. Endocrinol. Metab. 2008;93:1592–1599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

