Clinical efficacy of Danzhi Xiaoyao Powder in the treatment of post-stroke depression: A protocol for randomized, double-blind clinical study
- PMID: 34678863
- PMCID: PMC8542130
- DOI: 10.1097/MD.0000000000027318
Clinical efficacy of Danzhi Xiaoyao Powder in the treatment of post-stroke depression: A protocol for randomized, double-blind clinical study
Abstract
Background: Depression is a common complication after stroke and is closely related to the poor prognosis of stroke. Antidepressants are the priority drug in the treatment of post-stroke depression (PSD), but there are dependence and adverse reactions. Danzhi Xiaoyao Powder has a good effect on depression without obvious adverse reactions. At present, there is a lack of rigorous randomized controlled trials to evaluate the clinical efficacy of Danzhi Xiaoyao Powder in the treatment of PSD.
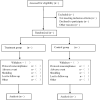
Methods: This is a prospective, randomized, double-blind, parallel controlled trial to explore the efficacy and safety of Danzhi Xiaoyao Powder in the treatment of PSD. The participants were randomly divided into treatment group and control group. The treatment group used Danzhi Xiaoyao Powder combined with escitalopram oxalate, and the control group used Danzhi Xiaoyao Powder simulant combined with citalopram oxalate. The two groups were both treated for 8 weeks and followed up for 3 months. Observational index includes: Total response rate, Hamilton depression scale, Barthel index, national institutes of health stroke scale, the modified Edinburgh-Scandinavian stroke scale, Incidence of adverse reactions. Finally, SPASS 22.0 software was used for statistical analysis of the data.
Discussion: This study will evaluate the clinical efficacy of Danzhi Xiaoyao Powder in the treatment of PSD. The results of this study will provide reliable evidence for the clinical use of Xiaoyao Powder in the treatment of PSD.
Trial registration: Open Science Framework Registration number: DOI 10.17605/OSF.IO/5V926.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
References
-
- Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol 2007;6:182–7. - PubMed
-
- Arseniou S, Arvaniti A, Samakouri M. Post-stroke depression: recognition and treatment interventions. Psychiatrike 2011;22:240–8. - PubMed
-
- Zhang GC, Fu WB, Xu NG, et al. . Meta analysis of the curative effect of acupuncture on post-stroke depression. Journal of traditional Chinese medicine 2012;32:06–11. - PubMed
-
- Ayerbe L, Ayis S, Wolfe CD, et al. . Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. The British journal of psychiatry: the journal of mental science 2013;202:14–21. - PubMed
-
- Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. International journal of stroke: official journal of the International Stroke Society 2014;9:1017–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical