Cortical Visual Impairment in Childhood: 'Blindsight' and the Sprague Effect Revisited
- PMID: 34679344
- PMCID: PMC8533908
- DOI: 10.3390/brainsci11101279
Cortical Visual Impairment in Childhood: 'Blindsight' and the Sprague Effect Revisited
Abstract
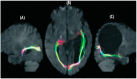
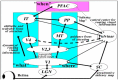
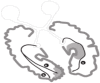
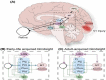
The paper discusses and provides support for diverse processes of brain plasticity in visual function after damage in infancy and childhood in comparison with injury that occurs in the adult brain. We provide support and description of neuroplastic mechanisms in childhood that do not seemingly exist in the same way in the adult brain. Examples include the ability to foster the development of thalamocortical connectivities that can circumvent the lesion and reach their cortical destination in the occipital cortex as the developing brain is more efficient in building new connections. Supporting this claim is the fact that in those with central visual field defects we can note that the extrastriatal visual connectivities are greater when a lesion occurs earlier in life as opposed to in the neurologically mature adult. The result is a significantly more optimized system of visual and spatial exploration within the 'blind' field of view. The discussion is provided within the context of "blindsight" and the "Sprague Effect".
Keywords: Sprague Effect; blindsight; cortical blindness; cortical visual impairment; infant vision; vision.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Khan R.I., O’Keefe M., Kenny D., Nolan L. Changing pattern of childhood blindness. Ir. Med. J. 2007;100:458–461. - PubMed
-
- National Institutes of Health. [(accessed on 11 September 2021)];2021 Available online: https://www.nei.nih.gov/about/news-and-events/news/vision-loss-children-....
Publication types
LinkOut - more resources
Full Text Sources

