Case Report of COVID-19 after Full Vaccination: Viral Loads and Anti-SARS-CoV-2 Antibodies
- PMID: 34679513
- PMCID: PMC8534434
- DOI: 10.3390/diagnostics11101815
Case Report of COVID-19 after Full Vaccination: Viral Loads and Anti-SARS-CoV-2 Antibodies
Abstract
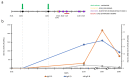
The introduction of effective vaccines against SARS-CoV-2 is expected to prevent COVID-19. However, sporadic cases of infection in vaccinated persons have been reported. We describe a case of a double-dose vaccinated woman with COVID-19. All stages of infection were observed, from no identification of virus, then the start of the infection, a high viral load, coming out of viraemia, and finally no detection of the virus. Despite the high viral load, the woman demonstrated mild COVID-19 symptoms, manifested only by a sore throat. The antibody results showed that she produced both post-infectious and post-vaccination immune responses. Phylogenetic analysis of the obtained viral genome sequence indicated that the virus belonged to the UK SARS-CoV-2 lineage B.1.1.7 (GR 501Y.V1; 20I/S:501Y.V1; Alpha variant).
Keywords: COVID-19; anti-SARS-CoV-2 antibodies; mRNA vaccine; viral loads.
Conflict of interest statement
None declared.
Figures
References
-
- World Health Organization Coronavirus Disease 2019. 2020. [(accessed on 23 March 2021)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
-
- Okba N.M., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., Lamers M.M., Sikkema R.S., De Bruin E., Chandler F.D., et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease 2019 patients. Emerg. Infect. Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

