Reliable Diagnostics of SARS-CoV-2 Infections Using One- and Two-Gene Molecular Tests for a Viral RNA Detection-Results Questioning Previous Observations
- PMID: 34679537
- PMCID: PMC8534906
- DOI: 10.3390/diagnostics11101839
Reliable Diagnostics of SARS-CoV-2 Infections Using One- and Two-Gene Molecular Tests for a Viral RNA Detection-Results Questioning Previous Observations
Abstract
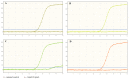
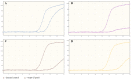
SARS-CoV-2 is a new virus from the Coronaviridae family and its rapid spread is now the most important medical problem worldwide. Currently used tests vary in the number and selection of SARS-CoV-2 target genes. Meanwhile, the choice of the appropriate target gene may be important in terms of a reliable detection of a viral RNA. As some researchers questioned the sensitivity of the monogenic VIASURE SARS-CoV-2 S gene Real Time PCR Detection Kit (CerTest Biotec, Zaragoza, Spain) in mid-2020, the aim of the study was to evaluate the usefulness of this kit, used along with the BD MAX™ System (Becton Dickinson, East Rutherford, NJ, USA), and compare the results with two-gene Bosphore Novel Coronavirus (2019-nCoV) Detection Kit v1 (Anatolia Diagnostics and Biotechnology Products Inc., Istanbul, Turkey). Both tests were carried out on 306 nasopharyngeal/oropharyngeal swabs. The consistent results (72 positive and 225 negative results found simultaneously in both kits) were obtained for 297 (97.1%) samples altogether, while discrepancies between the results of the evaluated tests were observed for nine (2.9%) specimens. There were no statistically significant differences between the method used and the frequency of positive results. Both tests, targeted at detecting one and two genes, are effective in SARS-CoV-2 RNA detection.
Keywords: 2019-nCoV; BD MAX™ System; COVID-19; E gene; ORF1ab gene; RNA detection; S gene; SARS-CoV-2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Lack of sensitivity of an IVD/CE-labelled kit targeting the S gene for detection of SARS-CoV-2.Clin Microbiol Infect. 2020 Oct;26(10):1417.e1-1417.e4. doi: 10.1016/j.cmi.2020.06.036. Epub 2020 Jul 8. Clin Microbiol Infect. 2020. PMID: 32652240 Free PMC article.
-
Analytical and clinical comparison of Viasure (CerTest Biotec) and 2019-nCoV CDC (IDT) RT-qPCR kits for SARS-CoV2 diagnosis.Virology. 2021 Jan 15;553:154-156. doi: 10.1016/j.virol.2020.10.010. Epub 2020 Nov 18. Virology. 2021. PMID: 33278737 Free PMC article.
-
Comparison of two real-time polymerase chain reaction assays for the detection of severe acute respiratory syndrome-CoV-2 from combined nasopharyngeal-throat swabs.Indian J Med Microbiol. 2020 Jul-Dec;38(3 & 4):385-389. doi: 10.4103/ijmm.IJMM_20_279. Indian J Med Microbiol. 2020. PMID: 33154251 Free PMC article.
-
Diagnosis with Metagenomic Next-Generation Sequencing (mNGS) technology and real-time PCR for SARS-CoV-2 Omicron detection using various nasopharyngeal swabs in SARS-CoV-2 Omicron.PLoS One. 2024 Aug 6;19(8):e0305289. doi: 10.1371/journal.pone.0305289. eCollection 2024. PLoS One. 2024. PMID: 39106263 Free PMC article.
-
Evaluation of Factors that Affect the Performance of COVID-19 Molecular Assays Including Presence of Symptoms, Number of Detected Genes and RNA Extraction Type.Mol Diagn Ther. 2022 Mar;26(2):229-238. doi: 10.1007/s40291-021-00574-y. Epub 2022 Jan 24. Mol Diagn Ther. 2022. PMID: 35072934 Free PMC article.
Cited by
-
Commercially available SARS-CoV-2 RT-qPCR diagnostic tests need obligatory internal validation.Sci Rep. 2023 Apr 28;13(1):6991. doi: 10.1038/s41598-023-34220-w. Sci Rep. 2023. PMID: 37117538 Free PMC article.
References
-
- World Health Organization Laboratory Testing for 2019 Novel Coronavirus (2019-NCoV) in Suspected Human Cases. [(accessed on 1 April 2021)]. Available online: https://www.who.int/publications-detail-redirect/10665-331501.
-
- Centers for Disease Control and Prevention Division of Viral Diseases CDC 2019-Novel Coronavirus (2019-NCoV) Real-Time RT-PCR Diagnostic Panel. [(accessed on 1 April 2021)]; Available online: https://www.fda.gov/media/134922/download.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous