Long-Term Monitoring of the Antibody Response to a SARS-CoV-2 Infection
- PMID: 34679613
- PMCID: PMC8534661
- DOI: 10.3390/diagnostics11101915
Long-Term Monitoring of the Antibody Response to a SARS-CoV-2 Infection
Abstract
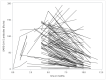
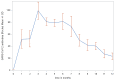
A group of 110 patients from the West Bohemian region who had been infected with COVID-19 was monitored for the purposes of this study. We focused on cases of mild or moderate COVID-19; statistically the most likely to occur. Day zero was defined as the day on which a positive PCR test was first established. The mean length of observation was 6.5 months, the maximum length 12 months. The first blood samples were taken from a smaller cohort during the 1-3 months following the first positive PCR test. We assumed that SARS-CoV-2 antibodies would be present during this period and therefore a limited number of samples were taken for the purpose of detecting antibodies. More samples were collected, starting 4 months after the first positive PCR test. A subsequent set of blood samples were drawn, mostly 6 months after the first ones. Our study confirmed the presence of total IgG SARS-CoV-2 antibodies up to 1 year after the onset of the disease. The peak of antibody production was observed in the third month after the first positive PCR test. A mathematical estimate of the median duration of antibody positivity was calculated to be 18 months from the onset of the COVID-19 infection.
Keywords: COVID-19; SARS-CoV-2; antibody; immunoassay; nucleocapsid protein; serological diagnostics; time period.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gozalbo-Rovira R., Gimenez E., Latorre V., Francés-Gómez C., Albert E., Buesa J., Marina A., Blasco M.L., Signes-Costa J., Rodríguez-Díaz J., et al. SARS-CoV-2 Antibodies, Serum Inflammatory Biomarkers and Clinical Severity of Hospitalized COVID-19 Patients. J. Clin. Virol. 2020;131:104611. doi: 10.1016/j.jcv.2020.104611. - DOI - PMC - PubMed
-
- Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.-B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous