Methods to Determine Chain-Breaking Antioxidant Activity of Nanomaterials beyond DPPH•. A Review
- PMID: 34679687
- PMCID: PMC8533328
- DOI: 10.3390/antiox10101551
Methods to Determine Chain-Breaking Antioxidant Activity of Nanomaterials beyond DPPH•. A Review
Abstract
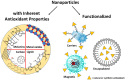
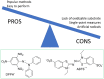
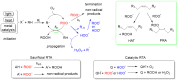
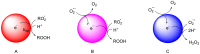
This review highlights the progress made in recent years in understanding the mechanism of action of nanomaterials with antioxidant activity and in the chemical methods used to evaluate their activity. Nanomaterials represent one of the most recent frontiers in the research for improved antioxidants, but further development is hampered by a poor characterization of the ''antioxidant activity'' property and by using oversimplified chemical methods. Inhibited autoxidation experiments provide valuable information about the interaction with the most important radicals involved in the lipid oxidation, namely alkylperoxyl and hydroperoxyl radicals, and demonstrate unambiguously the ability to stop the oxidation of organic materials. It is proposed that autoxidation methods should always complement (and possibly replace) the use of assays based on the quenching of stable radicals (such as DPPH• and ABTS•+). The mechanisms leading to the inhibition of the autoxidation (sacrificial and catalytic radical trapping antioxidant activity) are described in the context of nanoantioxidants. Guidelines for the selection of the appropriate testing conditions and of meaningful kinetic analysis are also given.
Keywords: ROS; antioxidant; assays; autoxidation; catalysis; nanoantioxidants; nanomaterial; oxygen; radicals; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Valgimigli L., Pratt D.A. Antioxidants in Chemistry and Biology. Encycl. Radic. Chem. Biol. Mater. 2012:1623–1677. doi: 10.1002/9781119953678.rad055. - DOI
-
- Medhe S., Bansal P., Srivastava M.M. Enhanced antioxidant activity of gold nanoparticle embedded 3,6-dihydroxyflavone: A combinational study. Appl. Nanosci. 2014;4:153–161. doi: 10.1007/s13204-012-0182-9. - DOI
-
- Viglianisi C., Scarlini A., Tofani L., Menichetti S., Baschieri A., Amorati R. Magnetic nanoantioxidants with improved radical-trapping stoichiometry as stabilizers for inhibition of peroxide formation in ethereal solvents. Sci. Rep. 2019;9:17219. doi: 10.1038/s41598-019-53531-5. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

