The Pattern of Blood-Milk Exchange for Antiparasitic Drugs in Dairy Ruminants
- PMID: 34679780
- PMCID: PMC8532883
- DOI: 10.3390/ani11102758
The Pattern of Blood-Milk Exchange for Antiparasitic Drugs in Dairy Ruminants
Abstract
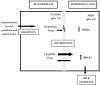
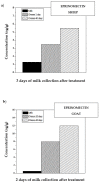
The prolonged persistence of milk residual concentration of different antiparasitic drugs in lactating dairy animals should be considered before recommending their use (label or extra-label) for parasite control in dairy animals. The partition blood-to-milk ratio for different antiparasitic compounds depends on their ability to diffuse across the mammary gland epithelium. The high lipophilicity of some of the most widely used antiparasitic drugs explains their high partition into milk and the extended persistence of high residual concentrations in milk after treatment. Most of the antiparasitic drug compounds studied were shown to be stable in various milk-related industrial processes. Thus, the levels of residues detected in raw milk can be directly applicable to estimating consumer exposure and dietary intake calculations when consuming heat-processed fluid milk. However, after milk is processed to obtain milk products such as cheese, yogurt, ricotta, and butter, the residues of lipophilic antiparasitic drugs are higher than those measured in the milk used for their elaboration. This review article contributes pharmacokinetics-based information, which is useful to understand the relevance of rational drug-based parasite control in lactating dairy ruminants to avoid undesirable consequences of residual drug concentrations in milk and derived products intended for human consumption.
Keywords: antiparasitic drugs; dairy animals; plasma–milk exchange; rational use in parasite control; residues in milk dairy products.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

