Telomere Length and Regulatory Genes as Novel Stress Biomarkers and Their Diversities in Broiler Chickens (Gallus gallus domesticus) Subjected to Corticosterone Feeding
- PMID: 34679783
- PMCID: PMC8532957
- DOI: 10.3390/ani11102759
Telomere Length and Regulatory Genes as Novel Stress Biomarkers and Their Diversities in Broiler Chickens (Gallus gallus domesticus) Subjected to Corticosterone Feeding
Abstract
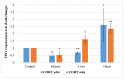
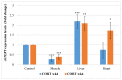
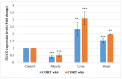
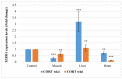
This study was designed to characterize telomere length and its regulatory genes and to evaluate their potential as well-being biomarkers. Chickens were fed a diet containing corticosterone (CORT) for 4 weeks and performances, organ weight, plasma CORT levels, telomere lengths and regulatory genes were measured and recorded. Body weights of CORT-fed chickens were significantly suppressed (p < 0.05), and organ weights and circulating CORT plasma levels (p < 0.05) were altered. Interaction effect of CORT and duration was significant (p < 0.05) on heart and liver telomere length. CORT significantly (p < 0.05) shortened the telomere length of the whole blood, muscle, liver and heart. The TRF1, chTERT, TELO2 and HSF1 were significantly (p < 0.05) upregulated in the liver and heart at week 4 although these genes and TERRA were downregulated in the muscles at weeks 2 and 4. Therefore, telomere lengths and their regulators are associated and diverse, so they can be used as novel biomarkers of stress in broiler chickens fed with CORT.
Keywords: broiler; corticosterone; performance; stress biomarkers; telomere regulators; telomeres.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rushen J. Problems associated with the interpretation of physiological data in the assessment of animal welfare. Appl. Anim. Behav. Sci. 1991;28:381–386. doi: 10.1016/0168-1591(91)90170-3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

