Sperm Repository for a Breeding Program of the Eastern Oyster Crassostrea virginica: Sample Collection, Processing, Cryopreservation, and Data Management Plan
- PMID: 34679857
- PMCID: PMC8532978
- DOI: 10.3390/ani11102836
Sperm Repository for a Breeding Program of the Eastern Oyster Crassostrea virginica: Sample Collection, Processing, Cryopreservation, and Data Management Plan
Abstract
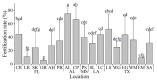
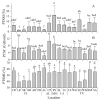
The Eastern oyster Crassostrea virginica (Family Ostreidae) is one of the most important fishery and aquaculture species in the U.S. and is a keystone species for coastal reefs. A breeding program was initiated in 2019 to support the fast-growing aquaculture industry culturing this species in the Gulf of Mexico. Oysters from 17 wild populations in embayment along the U.S. Gulf of Mexico coast from southwest Florida to the Matagorda Bay, Texas were used as broodstock for the program to maximize genetic diversity in the base population. A sperm repository of the broodstock was established to support the breeding project. The goal of this study was to demonstrate the sperm sample collection, processing, cryopreservation, and the data management plan involved in the establishment of a sperm germplasm repository of base populations. The supporting objectives were to: (1) develop a data management plan for the sperm repository; (2) streamline the procedure for sample collection, processing, and cryopreservation; (3) incorporate sperm quality analysis into the procedure, and (4) archive the cryopreserved samples as a repository for future use in the breeding program. This sperm repository included a total of 102 male oysters from the 17 collection sites (six oysters per site). A data management plan was developed with six categories, including sample collection, phenotype, fresh sperm, genotype, cryopreservation, and post-thaw sperm, as guide for data collection. Sperm collection was accomplished by strip spawn, and fresh sperm production, motility, and fertility were recorded for quality analysis. Cryopreserved sperm samples were sorted, labelled, archived, and stored in liquid nitrogen for future use. Post-thaw motility (1-30%) and plasm membrane integrity (15.34-70.36%) were recorded as post-thaw quality parameters. Overall, this study demonstrated a streamlined procedure of oyster sperm collection, processing, and cryopreservation for establishing a sperm repository that can serve as a template for construction of oyster germplasm repositories for breeding programs.
Keywords: Gulf of Mexico; aquaculture; breeding; cryopreservation; eastern oysters Crassostrea virginica; germplasm; sperm repository.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Olivier A.V., Jones L., Le Vay L., Christie M., Wilson J., Malham S.K. A global review of the ecosystem services provided by bivalve aquaculture. Rev. Aquac. 2020;12:3–25. doi: 10.1111/raq.12301. - DOI
-
- Galtsoff P.S. The American Oyster, Crassostrea Virginica Gmelin, Fishery Bulletin Volume 64. United States Government Printing Office; Washington, DC, USA: 1964. p. 458.
-
- MacKenzie C.L.J., Burrell V.G.J., Rosenfield A., Hobart W.L. The history, present condition, and future of the molluscan fisheries of North and Central America and Europe, Volume 1, Atlantic and Gulf Coasts. U.S. Dep. Commer. NOAA Tech. Rep. 1997;127:234.
-
- Schulte D.M. History of the Virginia oyster fishery, Chesapeake Bay, USA. Front. Mar. Sci. 2017;4:127. doi: 10.3389/fmars.2017.00127. - DOI
-
- Pine W.E., Walters C.J., Camp E.V., Bouchillon R., Ahrens R., Sturmer L., Berrigan M.E. The curious case of eastern oyster Crassostrea virginica stock status in Apalachicola Bay, Florida. Ecol. Soc. 2015;20:46. doi: 10.5751/ES-07827-200346. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

